A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer
- PMID: 32352498
- PMCID: PMC7193528
- DOI: 10.1001/jamaoncol.2020.0930
A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer
Abstract
Importance: Currently, there is no established second-line systemic treatment for biliary tract cancer (BTC). Preclinical data have demonstrated that the presence of tumor-infiltrating CD8 T cells and programmed cell death 1 ligand 1-expressing tumor cells in the tumor microenvironment of BTC supports the rationale of using programmed cell death 1 protein blockade immunotherapy in BTC.
Objective: To evaluate anticancer activity of nivolumab in patients with advanced refractory BTC.
Design, setting, and participants: In this single-group, multicenter phase 2 study of nivolumab, 54 patients with histologically confirmed BTC whose disease progressed while undergoing treatment with at least 1 line but no more than 3 lines of systemic therapy were enrolled between October 5, 2016, and December 26, 2018. Analysis was performed on an intention-to-treat basis.
Interventions: Nivolumab, 240 mg, was delivered intravenously every 2 weeks for 16 weeks, and then 480 mg was delivered intravenously every 4 weeks until disease progression or unacceptable toxic effects occurred.
Main outcomes and measures: The primary end point was investigator-assessed objective response rate, and the secondary end points were progression-free survival, overall survival, and incidence of adverse events.
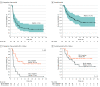
Results: A total of 54 patients (27 men and 27 women; median age, 65 years [range, 28-86 years]) enrolled, and 46 (22 men and 24 women; median age, 65 years [range, 28-86 years]) were examined for objective response with radiologic imaging. The investigator-assessed objective response rate was 22% (10 of 46), including 1 unconfirmed partial response, with a disease control rate of 59% (27 of 46). Central independent review found an objective response rate of 11% (5 of 46), including 1 unconfirmed partial response, with a disease control rate of 50% (23 of 46). All patients who responded to treated (hereafter referred to as responders) had mismatch repair protein-proficient tumors. The median duration of investigator-assessed response was not reached, with a median follow-up of 12.4 months. Among the intention-to-treat population, median progression-free survival was 3.68 months (95% CI, 2.30-5.69 months) and median overall survival was 14.24 months (95% CI, 5.98 months to not reached). Programmed cell death 1 ligand 1 expression in tumors was associated with prolonged progression-free survival (hazard ratio, 0.23; 95% CI, 0.10-0.51; P < .001). The most common treatment-related grade 3 or 4 toxic effects were hyponatremia (3 of 54 [6%]) and increased alkaline phosphatase (2 of 54 [4%]).
Conclusions and relevance: This study found that nivolumab was well tolerated and showed modest efficacy with durable response in patients with refractory BTC. Further studies are warranted to verify the findings and evaluate biomarkers for improved treatment selection for patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02829918.
Conflict of interest statement
Figures

Comment in
-
Need for More Comprehensive Evaluation of Immune-Related Adverse Events in Immunotherapy for Biliary Tract Cancer-Reply.JAMA Oncol. 2020 Dec 1;6(12):1981-1982. doi: 10.1001/jamaoncol.2020.4821. JAMA Oncol. 2020. PMID: 33090188 No abstract available.
-
Need for More Comprehensive Evaluation of Immune-Related Adverse Events in Immunotherapy for Biliary Tract Cancer.JAMA Oncol. 2020 Dec 1;6(12):1981. doi: 10.1001/jamaoncol.2020.4815. JAMA Oncol. 2020. PMID: 33090192 No abstract available.
References
-
- Lamarca A, Palmer DH, Wasan HS, Ross PJ, May YT, Arora A ABC-06: a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with oxaliplatin/5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced/metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. Presented at: 2019 ASCO Annual Meeting; June 2, 2019; Chicago, IL.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

