The connectome of the Caenorhabditis elegans pharynx
- PMID: 32352566
- PMCID: PMC7601127
- DOI: 10.1002/cne.24932
The connectome of the Caenorhabditis elegans pharynx
Abstract
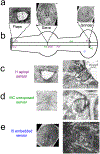
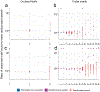
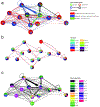
Detailed anatomical maps of individual organs and entire animals have served as invaluable entry points for ensuing dissection of their evolution, development, and function. The pharynx of the nematode Caenorhabditis elegans is a simple neuromuscular organ with a self-contained, autonomously acting nervous system, composed of 20 neurons that fall into 14 anatomically distinct types. Using serial electron micrograph (EM) reconstruction, we re-evaluate here the connectome of the pharyngeal nervous system, providing a novel and more detailed view of its structure and predicted function. Contrasting the previous classification of pharyngeal neurons into distinct inter- and motor neuron classes, we provide evidence that most pharyngeal neurons are also likely sensory neurons and most, if not all, pharyngeal neurons also classify as motor neurons. Together with the extensive cross-connectivity among pharyngeal neurons, which is more widespread than previously realized, the sensory-motor characteristics of most neurons define a shallow network architecture of the pharyngeal connectome. Network analysis reveals that the patterns of neuronal connections are organized into putative computational modules that reflect the known functional domains of the pharynx. Compared with the somatic nervous system, pharyngeal neurons both physically associate with a larger fraction of their neighbors and create synapses with a greater proportion of their neighbors. We speculate that the overall architecture of the pharyngeal nervous system may be reminiscent of the architecture of ancestral, primitive nervous systems.
Keywords: Caenorhabditis elegans; connectome.
© 2020 Wiley Periodicals, Inc.
Figures







References
-
- Albertson DG, & Thomson JN (1976). The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci, 275(938), 299–325. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dop... - PubMed
-
- Albertson DG, & Thomson JN (1976). The Pharynx of Caenorhabditis elegans. Phil. Trans. R. Soc. Lond. B Biol. Sci, 275(938), 299–325. - PubMed
-
- Avery L, & Horvitz HR (1989). Pharyngeal pumping continues after laser killing of the pharyngeal nervous system of C. elegans. Neuron, 3(1981), 473–485. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

