Amoxicillin-induced crystal nephropathy: A nationwide French pharmacovigilance databases study
- PMID: 32353167
- PMCID: PMC7576614
- DOI: 10.1111/bcp.14328
Amoxicillin-induced crystal nephropathy: A nationwide French pharmacovigilance databases study
Abstract
Aims: Amoxicillin (AMX)-induced crystal nephropathy (AICN) is a rarely reported adverse drug reaction (ADR) but its increase has been recently reported in the Paris area. Our aim was to investigate the incidence, characteristics and outcome of AICN in France.
Methods: Retrospective analysis of all AICN cases reported to the French National Pharmacovigilance Database and the Marketing Authorization Holders Pharmacovigilance Database. AICN notification rate was compared to intravenous AMX and AMX-clavulanate sales.
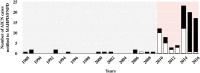
Results: In total, 101 AICN cases were included. Intravenous AMX/AMX-clavulanate was prescribed as surgical prophylaxis (32 surgical patients) or to treat infection (69 medical patients). AKI KDIGO stage 3 was observed in 70 patients and 24/70 patients required renal replacement therapy and/or intensive care unit admission. The annual notification rate of AICN was increased by a factor of 13 since 2010 (6 [0;7] and 77 [24;111] cases per 100 000 patient-years of exposure, before and after 2010 respectively; P < .001). In surgical patients, the increase in AICN has been reported since 2010 and was mainly related to inadequate AMX administration. In medical patients, the increase in AICN was observed since 2014. After 2014, medical patients were older (67 [42;77] vs 74 years [64;84] respectively; P < .05) and were treated more frequently for endocarditis (0/20 vs 15/49 respectively; P < .01). A contributing factor was observed or suspected in 62 patients.
Conclusion: AICN is a severe ADR that dramatically increased in France since 2010. Assessment of AICN contributing factors and AMX drug monitoring in patients receiving high dose of AMX could reduce the risk of AICN.
Keywords: acute kidney injury; adverse drug reaction; amoxicillin; crystals; renal replacement therapy.
© 2020 The British Pharmacological Society.
Conflict of interest statement
The authors have not disclosed any potential conflict of interest.
Figures


References
-
- World Health Organization . WHO report on surveillance of antibiotic consumption: 2016–2018 early implementation. https://www.who.int/medicines/areas/rational_use/who-amr-amc-report-2018.... Accessed January 21, 2020.
-
- Salvo F, De Sarro A, Caputi AP, Polimeni G. Amoxicillin and amoxicillin plus clavulanate: a safety review. Expert Opin Drug Saf. 2009;8(1):111‐118. - PubMed
-
- Fogazzi GB, Cantù M, Saglimbeni L, Daudon M. Amoxycillin, a rare but possible cause of crystalluria. Nephrol Dial Transplant. 2003;18(1):212‐214. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

