RNAi-Based Gene Therapy Rescues Developmental and Epileptic Encephalopathy in a Genetic Mouse Model
- PMID: 32353324
- PMCID: PMC7335739
- DOI: 10.1016/j.ymthe.2020.04.007
RNAi-Based Gene Therapy Rescues Developmental and Epileptic Encephalopathy in a Genetic Mouse Model
Abstract
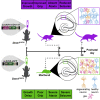
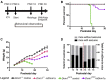
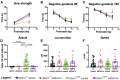
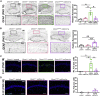
Developmental and epileptic encephalopathy (DEE) associated with de novo variants in the gene encoding dynamin-1 (DNM1) is a severe debilitating disease with no pharmacological remedy. Like most genetic DEEs, the majority of DNM1 patients suffer from therapy-resistant seizures and comorbidities such as intellectual disability, developmental delay, and hypotonia. We tested RNAi gene therapy in the Dnm1 fitful mouse model of DEE using a Dnm1-targeted therapeutic microRNA delivered by a self-complementary adeno-associated virus vector. Untreated or control-injected fitful mice have growth delay, severe ataxia, and lethal tonic-clonic seizures by 3 weeks of age. These major impairments are mitigated following a single treatment in newborn mice, along with key underlying cellular features including gliosis, cell death, and aberrant neuronal metabolic activity typically associated with recurrent seizures. Our results underscore the potential for RNAi gene therapy to treat DNM1 disease and other genetic DEEs where treatment would require inhibition of the pathogenic gene product.
Keywords: AAV9; DEE; DNM1; Lennox-Gastaut syndrome; RNA interferance; adeno-associated virus 9; developmental and epileptic encephalopathy; dynamin-1; epilepsy; gene therapy; infantile spasms.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Ferguson S.M., Brasnjo G., Hayashi M., Wölfel M., Collesi C., Giovedi S., Raimondi A., Gong L.W., Ariel P., Paradise S. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. - PubMed
-
- Marks B., Stowell M.H., Vallis Y., Mills I.G., Gibson A., Hopkins C.R., McMahon H.T. GTPase activity of dynamin and resulting conformation change are essential for endocytosis. Nature. 2001;410:231–235. - PubMed
-
- Powell K.A., Robinson P.J. Dephosphin/dynamin is a neuronal phosphoprotein concentrated in nerve terminals: evidence from rat cerebellum. Neuroscience. 1995;64:821–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

