The many faces of the anti-COVID immune response
- PMID: 32353870
- PMCID: PMC7191310
- DOI: 10.1084/jem.20200678
The many faces of the anti-COVID immune response
Abstract
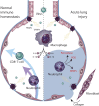
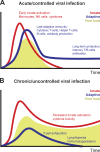
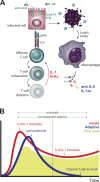
The novel 2019 strain of coronavirus is a source of profound morbidity and mortality worldwide. Compared with recent viral outbreaks, COVID-19 infection has a relatively high mortality rate, the reasons for which are not entirely clear. Furthermore, treatment options for COVID-19 infection are currently limited. In this Perspective, we explore the contributions of the innate and adaptive immune systems to both viral control as well as toxicity during COVID-19 infections and offer suggestions to both understand and therapeutically modulate anti-COVID immunity.
© 2020 Vardhana and Wolchok.
Conflict of interest statement
Disclosures: S.A. Vardhana reported personal fees from Immunai and personal fees from ADC Therapeutics outside the submitted work; in addition, S.A. Vardhana had a patent to PCT/US19/27610 pending. J.D. Wolchok reported personal fees from Tizona Pharmaceuticals, Adaptive Biotechnologies, Imvaq, Beigene, and Linneaus; and grants from AstraZeneca, Bristol Myers Squibb, and Sephora outside the submitted work. In addition, J.D. Wolchok had a patent to alphavirus replicon particles expressing TRP2 issued, a patent to Newcastle disease viruses for cancer therapy issued, a patent to xenogeneic DNA vaccines with royalties paid "Merial," a patent to myeloid-derived suppressor cell (MDSC) assay with royalties paid "Serametrix," a patent to anti-PD1 antibody licensed "Agenus," a patent to anti-CTLA4 antibodies licensed "Agenus," a patent to anti-GITR antibodies and methods of use thereof licensed "Agenus/Incyte," a patent to genomic signature to identify responders to ipilimumab in melanoma pending, a patent to engineered vaccinia viruses for cancer immunotherapy pending, a patent to anti-CD40 agonist mAb fused to monophosphoryl lipid A (MPL) for cancer therapy pending, a patent to CAR+ T cells targeting differentiation antigens as means to treat cancer pending, a patent to identifying and treating subjects at risk for checkpoint blockade therapy associated colitis pending, a patent to immunosuppressive follicular helper-like T cells modulated by immune checkpoint blockade pending, and a patent to phosphatidylserine targeting agents and uses thereof for adoptive T-cell therapies pending. J.D. Wolchok is a paid consultant for: Adaptive Biotech, Amgen, Apricity, Ascentage Pharma, Astellas, AstraZeneca, Bayer, Beigene, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly, Elucida, F Star, Imvaq, Janssen, Kyowa Hakko Kirin, Linneaus, Merck, Neon Therapuetics, Novaritis, Polynoma, Psioxus, Recepta, Takara Bio, Trieza, Truvax, Serametrix, Surface Oncology, Syndax, and Syntalogic.
Figures

References
-
- Ahmed R., Salmi A., Butler L.D., Chiller J.M., and Oldstone M.B.. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540. 10.1084/jem.160.2.521 - DOI - PMC - PubMed
-
- Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A., Jose J., Pinto R., Al-Omari A., Kharaba A., et al. ; Saudi Critical Care Trial Group . 2018. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 197:757–767. 10.1164/rccm.201706-1172OC - DOI - PubMed
-
- Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F., et al. . 2013. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 13:752–761. 10.1016/S1473-3099(13)70204-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

