Recent Advances in Studying Interfacial Adsorption of Bioengineered Monoclonal Antibodies
- PMID: 32353995
- PMCID: PMC7249052
- DOI: 10.3390/molecules25092047
Recent Advances in Studying Interfacial Adsorption of Bioengineered Monoclonal Antibodies
Abstract
Monoclonal antibodies (mAbs) are an important class of biotherapeutics; as of 2020, dozens are commercialized medicines, over a hundred are in clinical trials, and many more are in preclinical developmental stages. Therapeutic mAbs are sequence modified from the wild type IgG isoforms to varying extents and can have different intrinsic structural stability. For chronic treatments in particular, high concentration (≥ 100 mg/mL) aqueous formulations are often preferred for at-home administration with a syringe-based device. MAbs, like any globular protein, are amphiphilic and readily adsorb to interfaces, potentially causing structural deformation and even unfolding. Desorption of structurally perturbed mAbs is often hypothesized to promote aggregation, potentially leading to the formation of subvisible particles and visible precipitates. Since mAbs are exposed to numerous interfaces during biomanufacturing, storage and administration, many studies have examined mAb adsorption to different interfaces under various mitigation strategies. This review examines recent published literature focusing on adsorption of bioengineered mAbs under well-defined solution and surface conditions. The focus of this review is on understanding adsorption features driven by distinct antibody domains and on recent advances in establishing model interfaces suitable for high resolution surface measurements. Our summary highlights the need to further understand the relationship between mAb interfacial adsorption and desorption, solution aggregation, and product instability during fill-finish, transport, storage and administration.
Keywords: antibody; co-adsorption; mAbs; neutron reflection; self-assembly; structural unfolding; surface adsorption; surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

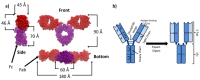
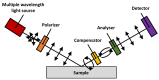
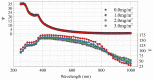

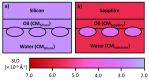
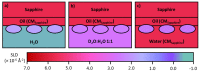
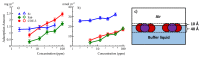
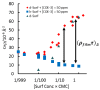

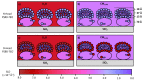
References
-
- Lu J.R., Zhao X., Yaseen M. Protein adsorption studied by neutron reflection. Curr. Opin. Colloid Interface Sci. 2007;12:9–16. doi: 10.1016/j.cocis.2007.02.001. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

