An LC-MS Assay to Measure Superoxide Radicals and Hydrogen Peroxide in the Blood System
- PMID: 32354089
- PMCID: PMC7280988
- DOI: 10.3390/metabo10050175
An LC-MS Assay to Measure Superoxide Radicals and Hydrogen Peroxide in the Blood System
Abstract
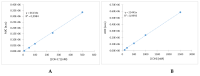
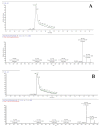
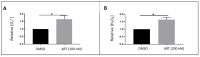
Red blood cells are constantly exposed to reactive species under physiological or pathological conditions or during administration of xenobiotics. Regardless of the source, its accurate quantification is paramount in the area of theragnostics, which had been elusive up until now. Even if there are a lot of approaches to evaluate the oxidative stress, very sensitive methods are missing for the blood system. We therefore sought to apply a highly sensitive approach, by liquid chromatography coupled to mass spectrometry (UPLC-MS), for the quantification of reactive species such as superoxide radical and hydrogen peroxide using dihydroethidium (DHE) and coumarin boronic acid (CBA) probes respectively through the detection of 2-hydroxyethidium (2OH-E+) and 7-hydroxycoumarin (COH). The use of the high-resolution mass spectrometry associated to UPLC ensured a selective detection of superoxide and hydrogen peroxide in the blood system under diverse conditions such as oxidized red blood cells (RBCs), untreated and treated parasitized RBCs. Moreover, this technique allowed the determination of reactive species in human plasma. This protocol provides a huge opportunity for in-depth study of several pathological conditions vis-a-vis their treatment in modern medicine.
Keywords: Plasmodium falciparum; human plasma; hydrogen peroxide species; liquid-chromatography; mass spectrometry; microvesicles; red blood cells; superoxide radicals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Santo A., Zhu H., Li Y.R. Free radicals: From health to disease. React. Oxyg. Species. 2016 doi: 10.20455/ros.2016.847. - DOI
LinkOut - more resources
Full Text Sources

