Cyclodextrin Prevents Abdominal Aortic Aneurysm via Activation of Vascular Smooth Muscle Cell Transcription Factor EB
- PMID: 32354235
- PMCID: PMC7606768
- DOI: 10.1161/CIRCULATIONAHA.119.044803
Cyclodextrin Prevents Abdominal Aortic Aneurysm via Activation of Vascular Smooth Muscle Cell Transcription Factor EB
Erratum in
-
Correction to: Cyclodextrin Prevents Abdominal Aortic Aneurysm via Activation of Vascular Smooth Muscle Cell Transcription Factor EB.Circulation. 2021 Jun 22;143(25):e1117. doi: 10.1161/CIR.0000000000000993. Epub 2021 Jun 21. Circulation. 2021. PMID: 34152797 No abstract available.
Abstract
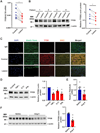
Background: Abdominal aortic aneurysm (AAA) is a severe aortic disease with a high mortality rate in the event of rupture. Pharmacological therapy is needed to inhibit AAA expansion and prevent aneurysm rupture. Transcription factor EB (TFEB), a master regulator of autophagy and lysosome biogenesis, is critical to maintain cell homeostasis. In this study, we aim to investigate the role of vascular smooth muscle cell (VSMC) TFEB in the development of AAA and establish TFEB as a novel target to treat AAA.
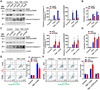
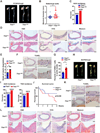
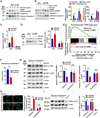
Methods: The expression of TFEB was measured in human and mouse aortic aneurysm samples. We used loss/gain-of-function approaches to understand the role of TFEB in VSMC survival and explored the underlying mechanisms through transcriptome and functional studies. Using VSMC-selective Tfeb knockout mice and different mouse AAA models, we determined the role of VSMC TFEB and a TFEB activator in AAA in vivo.
Results: We found that TFEB is downregulated in both human and mouse aortic aneurysm lesions. TFEB potently inhibits apoptosis in VSMCs, and transcriptome analysis revealed that TFEB regulates apoptotic signaling pathways, especially apoptosis inhibitor B-cell lymphoma 2. B-cell lymphoma 2 is significantly upregulated by TFEB and is required for TFEB to inhibit VSMC apoptosis. We consistently observed that TFEB deficiency increases VSMC apoptosis and promotes AAA formation in different mouse AAA models. Furthermore, we demonstrated that 2-hydroxypropyl-β-cyclodextrin, a clinical agent used to enhance the solubility of drugs, activates TFEB and inhibits AAA formation and progression in mice. Last, we found that 2-hydroxypropyl-β-cyclodextrin inhibits AAA in a VSMC TFEB-dependent manner in mouse models.
Conclusions: Our study demonstrated that TFEB protects against VSMC apoptosis and AAA. TFEB activation by 2-hydroxypropyl-β-cyclodextrin may be a promising therapeutic strategy for the prevention and treatment of AAA.
Keywords: aortic aneurysm, abdominal; apoptosis; autophagy; myocytes, smooth muscle.
Conflict of interest statement
Figures


References
-
- Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, Mastracci TM, Mell M, Murad MH, Nguyen LL, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67:2–77 e2. doi: 10.1016/j.jvs.2017.10.044 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

