Synthesis and evaluation of polyamine carbon quantum dots (CQDs) in Litopenaeus vannamei as a therapeutic agent against WSSV
- PMID: 32355276
- PMCID: PMC7192947
- DOI: 10.1038/s41598-020-64325-5
Synthesis and evaluation of polyamine carbon quantum dots (CQDs) in Litopenaeus vannamei as a therapeutic agent against WSSV
Abstract
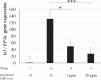
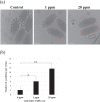
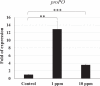
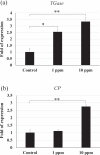
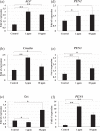
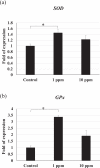
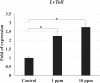
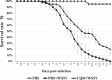
White spot syndrome virus (WSSV) is the causative agent of white spot syndrome (WSS), a disease that has led to severe mortality rates in cultured shrimp all over the world. The WSSV is a large, ellipsoid, enveloped double-stranded DNA virus with a wide host range among crustaceans. Currently, the main antiviral method is to block the receptor of the host cell membrane using recombinant viral proteins or virus antiserum. In addition to interference with the ligand-receptor binding, disrupting the structure of the virus envelope may also be a means to combat the viral infection. Carbon quantum dots (CQDs) are carbonaceous nanoparticles that have many advantageous characteristics, including small size, low cytotoxicity, cheap, and ease of production and modification. Polyamine-modified CQDs (polyamine CQDs) with strong antibacterial ability have been identified, previously. In this study, polyamine CQDs are shown to attach to the WSSV envelope and inhibit the virus infection, with a dose-dependent effect. The results also show that polyamine CQDs can upregulate several immune genes in shrimp and reduce the mortality upon WSSV infection. This is first study to identify that polyamine CQDs could against the virus. These results, indeed, provide a direction to develop effective antiviral strategies or therapeutic methods using polyamine CQDs in aquaculture.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Inhibition of White Spot Syndrome Virus (WSSV) in Pacific White Shrimp (Litopenaeus vannamei) Using Polyamine-Modified Carbon Quantum Dots.Methods Mol Biol. 2023;2610:67-73. doi: 10.1007/978-1-0716-2895-9_6. Methods Mol Biol. 2023. PMID: 36534282
-
Penaeidins restrict white spot syndrome virus infection by antagonizing the envelope proteins to block viral entry.Emerg Microbes Infect. 2020 Dec;9(1):390-412. doi: 10.1080/22221751.2020.1729068. Emerg Microbes Infect. 2020. PMID: 32397950 Free PMC article.
-
Evaluation on the antiviral activity of genipin against white spot syndrome virus in crayfish.Fish Shellfish Immunol. 2019 Oct;93:380-386. doi: 10.1016/j.fsi.2019.07.083. Epub 2019 Jul 30. Fish Shellfish Immunol. 2019. PMID: 31374312
-
Recent insights into host-pathogen interaction in white spot syndrome virus infected penaeid shrimp.J Fish Dis. 2015 Jul;38(7):599-612. doi: 10.1111/jfd.12279. Epub 2014 Jun 23. J Fish Dis. 2015. PMID: 24953507 Review.
-
Exploring the role of carbon quantum dots as countermeasure for SARS-CoV-2 virus.Virology. 2025 Feb;603:110339. doi: 10.1016/j.virol.2024.110339. Epub 2024 Dec 5. Virology. 2025. PMID: 39700784 Review.
Cited by
-
Enhanced antiviral defense against begomoviral infection in Nicotiana benthamiana through strategic utilization of fluorescent carbon quantum dots to activate plant immunity.J Nanobiotechnology. 2024 Nov 14;22(1):707. doi: 10.1186/s12951-024-02994-4. J Nanobiotechnology. 2024. PMID: 39543670 Free PMC article.
-
Graphene quantum dots as potential broad-spectrum antiviral agents.Nanoscale Adv. 2025 Jan 30;7(7):2032-2038. doi: 10.1039/d4na00879k. eCollection 2025 Mar 25. Nanoscale Adv. 2025. PMID: 39974343 Free PMC article.
-
Progress on Applying Carbon Dots for Inhibition of RNA Virus Infection.Nanotheranostics. 2022 Aug 21;6(4):436-450. doi: 10.7150/ntno.73918. eCollection 2022. Nanotheranostics. 2022. PMID: 36051856 Free PMC article. Review.
-
Quantum dots against SARS-CoV-2: diagnostic and therapeutic potentials.J Chem Technol Biotechnol. 2022 Jul;97(7):1640-1654. doi: 10.1002/jctb.7036. Epub 2022 Feb 4. J Chem Technol Biotechnol. 2022. PMID: 35463806 Free PMC article. Review.
-
You Don't Learn That in School: An Updated Practical Guide to Carbon Quantum Dots.Nanomaterials (Basel). 2021 Mar 1;11(3):611. doi: 10.3390/nano11030611. Nanomaterials (Basel). 2021. PMID: 33804394 Free PMC article. Review.
References
-
- Nakano H, et al. Mass mortalities of cultured kuruma shrimp, Penaeus japonicus, in japan in 1993: Epizootiological survey and infection trials. Fish Pathol. 1994;29:135–139. doi: 10.3147/jsfp.29.135. - DOI
-
- Wang YC, Lo CF, Chang PS, Kou GH. Experimental infection of white spot baculovirus in some cultured and wild decapods in Taiwan. Aquaculture. 1998;164:221–231. doi: 10.1016/S0044-8486(98)00188-4. - DOI
-
- Leu JH, et al. Whispovirus. Curr. Top. Microbiol. Immunol. 2009;328:197–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

