Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy
- PMID: 32355277
- PMCID: PMC7468577
- DOI: 10.1038/s41401-020-0414-6
Nanomedicines based on nanoscale metal-organic frameworks for cancer immunotherapy
Erratum in
-
Publisher Correction: 10.1038/s41401-020-0400-z,10.1038/s41401-020-0414-6,10.1038/s41401-020-0372-z.Acta Pharmacol Sin. 2021 May;42(5):844. doi: 10.1038/s41401-020-0464-9. Acta Pharmacol Sin. 2021. PMID: 32747717 Free PMC article. No abstract available.
Abstract
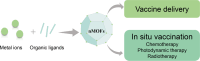
Cancer immunotherapy, with an aim to enhance host immune responses, has been recognized as a promising therapeutic treatment for cancer. A diversity of immunomodulatory agents, including tumor-associated antigens, adjuvants, cytokines and immunomodulators, has been explored for their ability to induce a cascading adaptive immune response. Nanoscale metal-organic frameworks (nMOFs), a class of crystalline-shaped nanomaterials formed by the self-assembly of organic ligands and metal nodes, are attractive for cancer immunotherapy because they feature tunable pore size, high surface area and loading capacity, and intrinsic biodegradability. In this review we summarize recent progress in the development of nMOFs for cancer immunotherapy, including cancer vaccine delivery and combination of in situ vaccination with immunomodulators to reverse immune suppression. Current challenges and future perspectives for rational design of nMOF-based cancer immunotherapy are also discussed.
Keywords: cancer immunotherapy; cancer vaccine; immune response; immunomodulators; in situ vaccination; nanoscale metal-organic frameworks (nMOFs).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

