Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury
- PMID: 32355959
- PMCID: PMC7534104
- DOI: 10.1093/brain/awaa052
Corticospinal-motor neuronal plasticity promotes exercise-mediated recovery in humans with spinal cord injury
Abstract
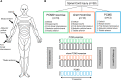
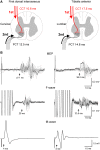
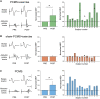
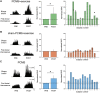
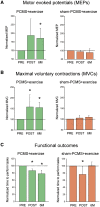
Rehabilitative exercise in humans with spinal cord injury aims to engage residual neural networks to improve functional recovery. We hypothesized that exercise combined with non-invasive stimulation targeting spinal synapses further promotes functional recovery. Twenty-five individuals with chronic incomplete cervical, thoracic, and lumbar spinal cord injury were randomly assigned to 10 sessions of exercise combined with paired corticospinal-motor neuronal stimulation (PCMS) or sham-PCMS. In an additional experiment, we tested the effect of PCMS without exercise in 13 individuals with spinal cord injury with similar characteristics. During PCMS, 180 pairs of stimuli were timed to have corticospinal volleys evoked by transcranial magnetic stimulation over the primary motor cortex arrive at corticospinal-motor neuronal synapses of upper- or lower-limb muscles (depending on the injury level), 1-2 ms before antidromic potentials were elicited in motor neurons by electrical stimulation of a peripheral nerve. Participants exercised for 45 min after all protocols. We found that the time to complete subcomponents of the Graded and Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) and the 10-m walk test decreased on average by 20% after all protocols. However, the amplitude of corticospinal responses elicited by transcranial magnetic stimulation and the magnitude of maximal voluntary contractions in targeted muscles increased on overage by 40-50% after PCMS combined or not with exercise but not after sham-PCMS combined with exercise. Notably, behavioural and physiological effects were preserved 6 months after the intervention in the group receiving exercise with PCMS but not in the group receiving exercise combined with sham-PCMS, suggesting that the stimulation contributed to preserve exercise gains. Our findings indicate that targeted non-invasive stimulation of spinal synapses might represent an effective strategy to facilitate exercise-mediated recovery in humans with different degrees of paralysis and levels of spinal cord injury.
Keywords: exercise training; maximal voluntary contraction; motor evoked potentials; neuromodulation; spinal plasticity.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Beekhuizen KS, Field-Fote EC.. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch Phys Med Rehabil 2008; 89: 602–8. - PubMed
-
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ.. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004; 42: 417–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

