Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease
- PMID: 32355963
- PMCID: PMC7241957
- DOI: 10.1093/brain/awaa090
Microglial exosomes facilitate α-synuclein transmission in Parkinson's disease
Abstract
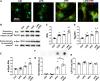
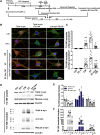
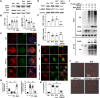
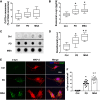
Accumulation of neuronal α-synuclein is a prominent feature in Parkinson's disease. More recently, such abnormal protein aggregation has been reported to spread from cell to cell and exosomes are considered as important mediators. The focus of such research, however, has been primarily in neurons. Given the increasing recognition of the importance of non-cell autonomous-mediated neurotoxicity, it is critical to investigate the contribution of glia to α-synuclein aggregation and spread. Microglia are the primary phagocytes in the brain and have been well-documented as inducers of neuroinflammation. How and to what extent microglia and their exosomes impact α-synuclein pathology has not been well delineated. We report here that when treated with human α-synuclein preformed fibrils, exosomes containing α-synuclein released by microglia are fully capable of inducing protein aggregation in the recipient neurons. Additionally, when combined with microglial proinflammatory cytokines, these exosomes further increased protein aggregation in neurons. Inhibition of exosome synthesis in microglia reduced α-synuclein transmission. The in vivo significance of these exosomes was demonstrated by stereotaxic injection of exosomes isolated from α-synuclein preformed fibrils treated microglia into the mouse striatum. Phosphorylated α-synuclein was observed in multiple brain regions consistent with their neuronal connectivity. These animals also exhibited neurodegeneration in the nigrostriatal pathway in a time-dependent manner. Depleting microglia in vivo dramatically suppressed the transmission of α-synuclein after stereotaxic injection of preformed fibrils. Mechanistically, we report here that α-synuclein preformed fibrils impaired autophagy flux by upregulating PELI1, which in turn, resulted in degradation of LAMP2 in activated microglia. More importantly, by purifying microglia/macrophage derived exosomes in the CSF of Parkinson's disease patients, we confirmed the presence of α-synuclein oligomer in CD11b+ exosomes, which were able to induce α-synuclein aggregation in neurons, further supporting the translational aspect of this study. Taken together, our study supports the view that microglial exosomes contribute to the progression of α-synuclein pathology and therefore, they may serve as a promising therapeutic target for Parkinson's disease.
Keywords: Parkinson’s disease; exosome; microglia; transmission; α-synuclein.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures




Similar articles
-
Reactive microglia enhance the transmission of exosomal α-synuclein via toll-like receptor 2.Brain. 2021 Aug 17;144(7):2024-2037. doi: 10.1093/brain/awab122. Brain. 2021. PMID: 33792662
-
Exosome-mediated microglia-astrocyte interactions drive neuroinflammation in Parkinson's disease with Peli1 as a potential therapeutic target.Pharmacol Res. 2025 Sep;219:107908. doi: 10.1016/j.phrs.2025.107908. Epub 2025 Aug 13. Pharmacol Res. 2025. PMID: 40816423
-
Plasma exosomes impair microglial degradation of α-synuclein through V-ATPase subunit V1G1.CNS Neurosci Ther. 2024 May;30(5):e14738. doi: 10.1111/cns.14738. CNS Neurosci Ther. 2024. PMID: 38702933 Free PMC article.
-
From inflammasome to Parkinson's disease: Does the NLRP3 inflammasome facilitate exosome secretion and exosomal alpha-synuclein transmission in Parkinson's disease?Exp Neurol. 2021 Feb;336:113525. doi: 10.1016/j.expneurol.2020.113525. Epub 2020 Nov 5. Exp Neurol. 2021. PMID: 33161049 Review.
-
Activated microglia facilitate the transmission of α-synuclein in Parkinson's disease.Neurochem Int. 2021 Sep;148:105094. doi: 10.1016/j.neuint.2021.105094. Epub 2021 Jun 8. Neurochem Int. 2021. PMID: 34097990 Review.
Cited by
-
Transcriptome Analysis Unveils That Exosomes Derived from M1-Polarized Microglia Induce Ferroptosis of Neuronal Cells.Cells. 2022 Dec 7;11(24):3956. doi: 10.3390/cells11243956. Cells. 2022. PMID: 36552720 Free PMC article.
-
Exosome: The "Off-the-Shelf" Cellular Nanocomponent as a Potential Pathogenic Agent, a Disease Biomarker, and Neurotherapeutics.Front Pharmacol. 2022 May 24;13:878058. doi: 10.3389/fphar.2022.878058. eCollection 2022. Front Pharmacol. 2022. PMID: 35685643 Free PMC article. Review.
-
Role of Glia-Derived Extracellular Vesicles in Neurodegenerative Diseases.Front Aging Neurosci. 2021 Oct 20;13:765395. doi: 10.3389/fnagi.2021.765395. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34744700 Free PMC article. Review.
-
Linking α-synuclein-induced synaptopathy and neural network dysfunction in early Parkinson's disease.Brain Commun. 2022 Jun 22;4(4):fcac165. doi: 10.1093/braincomms/fcac165. eCollection 2022. Brain Commun. 2022. PMID: 35822101 Free PMC article. Review.
-
Border-associated macrophages in the central nervous system.J Neuroinflammation. 2024 Mar 13;21(1):67. doi: 10.1186/s12974-024-03059-x. J Neuroinflammation. 2024. PMID: 38481312 Free PMC article. Review.
References
-
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, et al.Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol 1997; 12: 25–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

