Delivering Clinical impacts of the MRI diagnostic pathway in prostate cancer diagnosis
- PMID: 32356003
- PMCID: PMC7716818
- DOI: 10.1007/s00261-020-02547-x
Delivering Clinical impacts of the MRI diagnostic pathway in prostate cancer diagnosis
Abstract
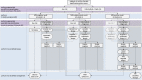
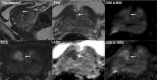
Pre-biopsy multiparametric MRI is now recommended by multiple guidelines, not only for men with persistent suspicion of prostate cancer after prior negative systematic biopsy, but also at initial screening before the first biopsy. The major benefit of pre-biopsy MRI in the diagnostic work-up is to promote individualized risk-adapted approaches for biopsy-decision management. Multiple MRI-directed diagnostic pathways can be conceived, with each approach having net-benefit trade-offs between benefits and harms, based on improved diagnostic yields of significant cancers and reduced biopsy testing and reduced detection of indolent prostate cancer. In this paper, we illustrate how clinical benefits can be maximized in men with MRI-negative and MRI-positive results, using the PI-RADS Multiparametric MRI and MRI-directed biopsy pathway. From a practice perspective, we emphasize five golden rules: (1) that multiparametric MRI approach including targeted biopsies be reserved for men likely to benefit from early detection and treatment of prostate cancer; (2) that there is a need to carefully assess risk of significant disease using PSA and clinical parameters before and after MRI; (3) do not offer immediate biopsy if the MRI is negative, unless other high-risk factors are present; (4) accept that not all significant cancers are found immediately and have robust 'safety nets' for men with negative MRI scans who avoid immediate biopsy and for positive MRI patients with negative or non-explanatory histology; and (5) use MRI-directed biopsy methods that minimize overdiagnosis and improve risk stratification.
Keywords: Biopsy; Magnetic resonance imaging (MRI); Multivariate risk prediction; Nomogram; Prostate cancer; Risk calculator; Risk stratification.
Conflict of interest statement
The authors have no potential conflict of interest to declare.
Figures





References
-
- Mohler JL, Antonarakis ES, Armstrong AJ, D'Amico AV, Davis BJ, Dorff T, Eastham JA, Enke CA, Farrington TA, Higano CS, Horwitz EM, Hurwitz M, Ippolito JE, Kane CJ, Kuettel MR, Lang JM, McKenney J, Netto G, Penson DF, Plimack ER, Pow-Sang JM, Pugh TJ, Richey S, Roach M, Rosenfeld S, Schaeffer E, Shabsigh A, Small EJ, Spratt DE, Srinivas S, Tward J, Shead DA, Freedman-Cass DA. Prostate Cancer, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(5):479–505. doi: 10.6004/jnccn.2019.0023. - DOI - PubMed
-
- Bjurlin MA, Carroll PR, Eggener S, Fulgham PF, Margolis DJ, Pinto PA, Rosenkrantz AB, Rubenstein JN, Rukstalis DB, Taneja SS, Turkbey B. Update of the AUA Policy Statement on the Use of Multiparametric Magnetic Resonance Imaging in the Diagnosis, Staging and Management of Prostate Cancer. The Journal of urology. 2019;23(10):0000000000000617. - PMC - PubMed
-
- Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, Fossati N, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, Matveev VB, Moldovan PC, van den Bergh RCN, Van den Broeck T, van der Poel HG, van der Kwast TH, Rouviere O, Schoots IG, Wiegel T, Cornford P (2020) EAU-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. https://uroweb.org/guideline/prostate-cancer/. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

