QSAR without borders
- PMID: 32356548
- PMCID: PMC8008490
- DOI: 10.1039/d0cs00098a
QSAR without borders
Erratum in
-
Correction: QSAR without borders.Chem Soc Rev. 2020 Jun 8;49(11):3716. doi: 10.1039/d0cs90041a. Chem Soc Rev. 2020. PMID: 32441715
Abstract
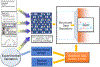
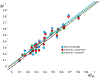
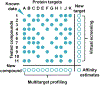
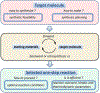
Prediction of chemical bioactivity and physical properties has been one of the most important applications of statistical and more recently, machine learning and artificial intelligence methods in chemical sciences. This field of research, broadly known as quantitative structure-activity relationships (QSAR) modeling, has developed many important algorithms and has found a broad range of applications in physical organic and medicinal chemistry in the past 55+ years. This Perspective summarizes recent technological advances in QSAR modeling but it also highlights the applicability of algorithms, modeling methods, and validation practices developed in QSAR to a wide range of research areas outside of traditional QSAR boundaries including synthesis planning, nanotechnology, materials science, biomaterials, and clinical informatics. As modern research methods generate rapidly increasing amounts of data, the knowledge of robust data-driven modelling methods professed within the QSAR field can become essential for scientists working both within and outside of chemical research. We hope that this contribution highlighting the generalizable components of QSAR modeling will serve to address this challenge.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures





References
-
- Hansch C, Maloney P, Fujita T and Muir R, Nature, 1962, 194, 178–180.
-
- Ban F, Dalal K, Li H, LeBlanc E, Rennie PS and Cherkasov A, J. Chem. Inf. Model, 2017, 57, 1018–1028. - PubMed
-
- Simón-Vidal L, García-Calvo O, Oteo U, Arrasate S, Lete E, Sotomayor N and González-Díaz H, J. Chem. Inf. Model, 2018, 58, 1384–1396. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

