Developmental underpinnings of spermatogonial stem cell establishment
- PMID: 32356598
- PMCID: PMC8324036
- DOI: 10.1111/andr.12810
Developmental underpinnings of spermatogonial stem cell establishment
Abstract
Background: The germline serves as a conduit for transmission of genetic and epigenetic information from one generation to the next. In males, spermatozoa are the final carriers of inheritance and their continual production is supported by a foundational population of spermatogonial stem cells (SSCs) that forms from prospermatogonial precursors during the early stages of neonatal development. In mammals, the timing for which SSCs are specified and the underlying mechanisms guiding this process remain to be completely understood.
Objectives: To propose an evolving concept for how the foundational SSC population is established.
Materials and methods: This review summarizes recent and historical findings from peer-reviewed publications made primarily with mouse models while incorporating limited studies from humans and livestock.
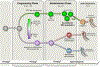
Results and conclusion: Establishment of the SSC population appears to follow a biphasic pattern involving a period of fate programming followed by an establishment phase that culminates in formation of the SSC population. This model for establishment of the foundational SSC population from precursors is anticipated to extend across mammalian species and include humans and livestock, albeit on different timescales.
Keywords: establishment; germline; gonocyte; prospermatogonia; specification; spermatogonial stem cel.
© 2020 American Society of Andrology and European Academy of Andrology.
Figures
References
-
- Santos AC, Lehmann R. Germ cell specification and migration in Drosophila and beyond. Curr Biol. 2004;14(14):R578–R589. - PubMed
-
- Ohinata Y, Payer B, O’Carroll D, et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature. 2005;436(7048):207–213. - PubMed
-
- Leitch HG, Tang WW, Surani MA. Primordial germ-cell development and epigenetic reprogramming in mammals. Curr Top Dev Biol. 2013;104:149–187. - PubMed
-
- Magnusdottir E, Surani MA. How to make a primordial germ cell. Development. 2014;141(2):245–252. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

