Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset
- PMID: 32356867
- PMCID: PMC7195694
- DOI: 10.1001/jamainternmed.2020.2020
Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset
Erratum in
-
Errors in Abstract, Text, and Supplement.JAMA Intern Med. 2020 Sep 1;180(9):1264. doi: 10.1001/jamainternmed.2020.4924. JAMA Intern Med. 2020. PMID: 32897379 Free PMC article. No abstract available.
Abstract
Importance: The dynamics of coronavirus disease 2019 (COVID-19) transmissibility are yet to be fully understood. Better understanding of the transmission dynamics is important for the development and evaluation of effective control policies.
Objective: To delineate the transmission dynamics of COVID-19 and evaluate the transmission risk at different exposure window periods before and after symptom onset.
Design, setting, and participants: This prospective case-ascertained study in Taiwan included laboratory-confirmed cases of COVID-19 and their contacts. The study period was from January 15 to March 18, 2020. All close contacts were quarantined at home for 14 days after their last exposure to the index case. During the quarantine period, any relevant symptoms (fever, cough, or other respiratory symptoms) of contacts triggered a COVID-19 test. The final follow-up date was April 2, 2020.
Main outcomes and measures: Secondary clinical attack rate (considering symptomatic cases only) for different exposure time windows of the index cases and for different exposure settings (such as household, family, and health care).
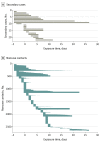
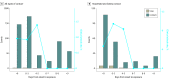
Results: We enrolled 100 confirmed patients, with a median age of 44 years (range, 11-88 years), including 44 men and 56 women. Among their 2761 close contacts, there were 22 paired index-secondary cases. The overall secondary clinical attack rate was 0.7% (95% CI, 0.4%-1.0%). The attack rate was higher among the 1818 contacts whose exposure to index cases started within 5 days of symptom onset (1.0% [95% CI, 0.6%-1.6%]) compared with those who were exposed later (0 cases from 852 contacts; 95% CI, 0%-0.4%). The 299 contacts with exclusive presymptomatic exposures were also at risk (attack rate, 0.7% [95% CI, 0.2%-2.4%]). The attack rate was higher among household (4.6% [95% CI, 2.3%-9.3%]) and nonhousehold (5.3% [95% CI, 2.1%-12.8%]) family contacts than that in health care or other settings. The attack rates were higher among those aged 40 to 59 years (1.1% [95% CI, 0.6%-2.1%]) and those aged 60 years and older (0.9% [95% CI, 0.3%-2.6%]).
Conclusions and relevance: In this study, high transmissibility of COVID-19 before and immediately after symptom onset suggests that finding and isolating symptomatic patients alone may not suffice to contain the epidemic, and more generalized measures may be required, such as social distancing.
Conflict of interest statement
Figures
Comment in
-
Contact Tracing, Testing, and Control of COVID-19-Learning From Taiwan.JAMA Intern Med. 2020 Sep 1;180(9):1163-1164. doi: 10.1001/jamainternmed.2020.2072. JAMA Intern Med. 2020. PMID: 32356871 No abstract available.
-
COVID-19 Transmission Conclusions Justified by the Analysis Results?JAMA Intern Med. 2020 Sep 1;180(9):1262. doi: 10.1001/jamainternmed.2020.4097. JAMA Intern Med. 2020. PMID: 32897381 No abstract available.
References
-
- World Health Organization Coronavirus disease 2019 (COVID-19) situation report—48. Published March 8, 2020. Accessed April 5, 2020. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

