Effect of stevia on the gut microbiota and glucose tolerance in a murine model of diet-induced obesity
- PMID: 32356872
- PMCID: PMC7233940
- DOI: 10.1093/femsec/fiaa079
Effect of stevia on the gut microbiota and glucose tolerance in a murine model of diet-induced obesity
Abstract
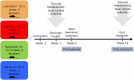
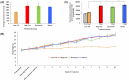
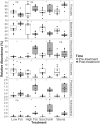
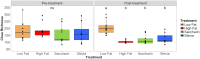
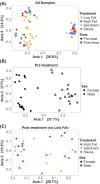
Artificial sweeteners have been shown to induce glucose intolerance by altering the gut microbiota; however, little is known about the effect of stevia. Here, we investigate whether stevia supplementation induces glucose intolerance by altering the gut microbiota in mice, hypothesizing that stevia would correct high fat diet-induced glucose intolerance and alter the gut microbiota. Mice were split into four treatment groups: low fat, high fat, high fat + saccharin and high fat + stevia. After 10 weeks of treatment, mice consuming a high fat diet (60% kcal from fat) developed glucose intolerance and gained more weight than mice consuming a low fat diet. Stevia supplementation did not impact body weight or glucose intolerance. Differences in species richness and relative abundances of several phyla were observed in low fat groups compared to high fat, stevia and saccharin. We identified two operational taxonomic groups that contributed to differences in beta-diversity between the stevia and saccharin groups: Lactococcus and Akkermansia in females and Lactococcus in males. Our results demonstrate that stevia does not rescue high fat diet-induced changes in glucose tolerance or the microbiota, and that stevia results in similar alterations to the gut microbiota as saccharin when administered in concordance with a high fat diet.
Keywords: Lactococcus; 16S rRNA; PERMANOVA; SIMPER; glucose tolerance; gut microbiota; saccharin.
© FEMS 2020.
Figures