Autophagosome biogenesis: From membrane growth to closure
- PMID: 32357219
- PMCID: PMC7265318
- DOI: 10.1083/jcb.202002085
Autophagosome biogenesis: From membrane growth to closure
Abstract
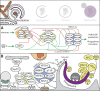
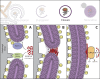
Autophagosome biogenesis involves de novo formation of a membrane that elongates to sequester cytoplasmic cargo and closes to form a double-membrane vesicle (an autophagosome). This process has remained enigmatic since its initial discovery >50 yr ago, but our understanding of the mechanisms involved in autophagosome biogenesis has increased substantially during the last 20 yr. Several key questions do remain open, however, including, What determines the site of autophagosome nucleation? What is the origin and lipid composition of the autophagosome membrane? How is cargo sequestration regulated under nonselective and selective types of autophagy? This review provides key insight into the core molecular mechanisms underlying autophagosome biogenesis, with a specific emphasis on membrane modeling events, and highlights recent conceptual advances in the field.
© 2020 Melia et al.
Figures


References
-
- Alemu E.A., Lamark T., Torgersen K.M., Birgisdottir A.B., Larsen K.B., Jain A., Olsvik H., Øvervatn A., Kirkin V., and Johansen T.. 2012. ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 287:39275–39290. 10.1074/jbc.M112.378109 - DOI - PMC - PubMed
-
- Andrejeva G., Gowan S., Lin G., Wong Te Fong A.L., Shamsaei E., Parkes H.G., Mui J., Raynaud F.I., Asad Y., Vizcay-Barrena G., et al. . 2019. De novo phosphatidylcholine synthesis is required for autophagosome membrane formation and maintenance during autophagy. Autophagy. 10.1080/15548627.2019.1659608 - DOI - PMC - PubMed

