Treatment of Acutely Ruptured Cerebral Aneurysms With the Woven EndoBridge Device: Experience Post-FDA Approval
- PMID: 32357228
- PMCID: PMC8929032
- DOI: 10.1093/neuros/nyaa092
Treatment of Acutely Ruptured Cerebral Aneurysms With the Woven EndoBridge Device: Experience Post-FDA Approval
Abstract
Background: Coil embolization of ruptured bifurcation aneurysms is challenging and often necessitates adjunctive stenting, which requires antiplatelet therapy in the setting of subarachnoid hemorrhage (SAH). The Woven EndoBridge (WEB; Terumo) device is an alternative self-expanding 3D mesh that does not require antiplatelet agents. However, its use has been mostly reserved for unruptured aneurysms.
Objective: To assess the safety and feasibility of ruptured aneurysm treatment with the WEB.
Methods: Retrospective analysis of 9 SAH patients with 11 aneurysms that were treated with the WEB device at 2 institutions after FDA approval.
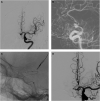
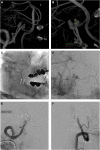
Results: Hunt and Hess grades were III and IV in 4 (44%) each and V in 1 (11%). All patients were treated within 24 h of hospitalization, and a single WEB was used in all but one aneurysm. Aneurysms treated were 3 basilar tip, 2 anterior communicating artery, 2 posterior inferior cerebellarartery, 1 middle cerebral artery, 1 carotid-ophthalmic artery, 1 posterior communicating artery, and 1 vertebrobasilar junction. Mean aneurysm height and width were 6.2 ± 2.2 mm (range: 3-10) and 5.6 ± 3.0 mm (range: 3.3-14), respectively. Mean dome-to-neck ratio was 1.7 ± 0.8 (range: 1.0-3.8). There was one intraoperative rupture that occurred because of device dislodgement and was managed with embolization. There were no treatment-related mortalities and no re-rupture after securement of the aneurysms with the WEB.
Conclusion: Our preliminary experience indicates that the WEB device can be used safely for ruptured aneurysms of various sizes in the anterior and posterior circulation. Larger series with long-term follow-up are necessary to confirm our findings.
Keywords: Cerebral aneurysm; Embolization; Endovascular; Subarachnoid hemorrhage; WEB device.
Copyright © 2020 by the Congress of Neurological Surgeons.
Figures

References
-
- Molyneux A, Kerr R, Stratton Iet al. .. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274. - PubMed
-
- McDougall CG, Spetzler RF, Zabramski JMet al. .. The barrow ruptured aneurysm trial. J Neurosurg. 2012;116(1):135-144. - PubMed
-
- Li H, Pan R, Wang Het al. .. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013;44(1):29-37. - PubMed
-
- Cognard C, Pierot L, Anxionnat R, Ricolfi F, Clarity Study G. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: clinical results of the clarity GDC study. Neurosurgery. 2011;69(4):837-841; discussion 842. - PubMed
-
- FDA . Woven EndoBridge (WEB) Aneurysm Embolization System - P170032. https://www.fda.gov/medical-devices/recently-approved-devices/woven-endo.... Accessed July 31, 2019.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

