The Product Specificities of Maize Terpene Synthases TPS4 and TPS10 Are Determined both by Active Site Amino Acids and Residues Adjacent to the Active Site
- PMID: 32357450
- PMCID: PMC7284416
- DOI: 10.3390/plants9050552
The Product Specificities of Maize Terpene Synthases TPS4 and TPS10 Are Determined both by Active Site Amino Acids and Residues Adjacent to the Active Site
Abstract
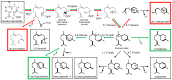
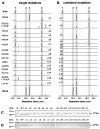
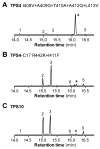
Terpene synthases make up a large family of enzymes that convert prenyl diphosphates into an enormous variety of terpene skeletons. Due to their electrophilic reaction mechanism-which involves the formation of carbocations followed by hydride shifts and skeletal rearrangements-terpene synthases often produce complex mixtures of products. In the present study, we investigate amino acids that determine the product specificities of the maize terpene synthases TPS4 and TPS10. The enzymes showed 57% amino acid similarity and produced different mixtures of sesquiterpenes. Sequence comparisons and structure modeling revealed that out of the 43 amino acids forming the active site cavity, 17 differed between TPS4 and TPS10. While combined mutation of these 17 residues in TPS4 resulted in an enzyme with a product specificity similar to TPS10, the additional mutation of two amino acids next to the active site led to a nearly complete conversion of TPS4 into TPS10. These data demonstrate that the different product specificities of TPS4 and TPS10 are determined not only by amino acids forming the active site cavity, but also by neighboring residues that influence the conformation of active site amino acids.
Keywords: 7-epi-sesquithujene; Zea mays; sesquiterpene synthase; site-directed mutagenesis; terpene synthase reaction mechanism; α-bergamotene; β-bisabolene; β-farnesene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Köllner T.G., Schnee C., Gershenzon J., Degenhardt J. The variability of sesquiterpenes emitted from two Zea mays cultivars is controlled by allelic variation of two terpene synthase genes encoding stereoselective multiple product enzymes. Plant Cell. 2004;16:1115–1131. doi: 10.1105/tpc.019877. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

