Characterization of Endoplasmic Reticulum (ER) in Human Pluripotent Stem Cells Revealed Increased Susceptibility to Cell Death upon ER Stress
- PMID: 32357563
- PMCID: PMC7291192
- DOI: 10.3390/cells9051078
Characterization of Endoplasmic Reticulum (ER) in Human Pluripotent Stem Cells Revealed Increased Susceptibility to Cell Death upon ER Stress
Abstract
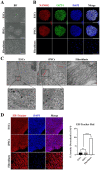
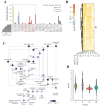
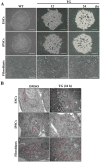
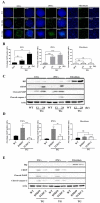
Human pluripotent stem cells (hPSCs), such as embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), have a well-orchestrated program for differentiation and self-renewal. However, the structural features of unique proteostatic-maintaining mechanisms in hPSCs and their features, distinct from those of differentiated cells, in response to cellular stress remain unclear. We evaluated and compared the morphological features and stress response of hPSCs and fibroblasts. Compared to fibroblasts, electron microscopy showed simpler/fewer structures with fewer networks in the endoplasmic reticulum (ER) of hPSCs, as well as lower expression of ER-related genes according to meta-analysis. As hPSCs contain low levels of binding immunoglobulin protein (BiP), an ER chaperone, thapsigargin treatment sharply increased the gene expression of the unfolded protein response. Thus, hPSCs with decreased chaperone function reacted sensitively to ER stress and entered apoptosis faster than fibroblasts. Such ER stress-induced apoptotic processes were abolished by tauroursodeoxycholic acid, an ER-stress reliever. Hence, our results revealed that as PSCs have an underdeveloped structure and express fewer BiP chaperone proteins than somatic cells, they are more susceptible to ER stress-induced apoptosis in response to stress.
Keywords: C/EBP homologous protein (CHOP); ER stress; binding immunoglobulin protein (BiP); endoplasmic reticulum (ER); human pluripotent stem cells; proteostasis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

