Autologous bone marrow-derived mononuclear cell therapy in three patients with severe asthma
- PMID: 32357905
- PMCID: PMC7193384
- DOI: 10.1186/s13287-020-01675-x
Autologous bone marrow-derived mononuclear cell therapy in three patients with severe asthma
Abstract
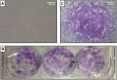
Background: Despite recent advances in understanding its pathophysiology and development of novel therapies, asthma remains a serious public health issue worldwide. Combination therapy with inhaled corticosteroids and long-acting β2-adrenoceptor agonists results in disease control for many patients, but those who exhibit severe asthma are often unresponsive to conventional treatment, experiencing worse quality of life, frequent exacerbations, and increasing healthcare costs. Bone marrow-derived mononuclear cell (BMMC) transplantation has been shown to reduce airway inflammation and remodeling and improve lung function in experimental models of allergic asthma.
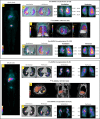
Methods: This is a case series of three patients who presented severe asthma, unresponsive to conventional therapy and omalizumab. They received a single intravenous dose of autologous BMMCs (2 × 107) and were periodically evaluated for 1 year after the procedure. Endpoint assessments included physical examination, quality of life questionnaires, imaging (computed tomography, single-photon emission computed tomography, and ventilation/perfusion scan), lung function tests, and a 6-min walk test.
Results: All patients completed the follow-up protocol. No serious adverse events attributable to BMMC transplantation were observed during or after the procedure. Lung function remained stable throughout. A slight increase in ventilation of the right lung was observed on day 120 after BMMC transplantation in one patient. All three patients reported improvement in quality of life in the early post-procedure course.
Conclusions: This paper described for the first time the effects of BMMC therapy in patients with severe asthma, providing a basis for subsequent trials to assess the efficacy of this therapy.
Keywords: Asthma; Autologous transplantation; Bone marrow mononuclear cells; Cell therapy; Lung.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Global Initiative for Asthma. Global strategy for asthma management and prevention; 2019. Available at https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-J.... Accessed 15 Jan 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

