Neuronal and glial regulation of CNS angiogenesis and barriergenesis
- PMID: 32358096
- PMCID: PMC7197727
- DOI: 10.1242/dev.182279
Neuronal and glial regulation of CNS angiogenesis and barriergenesis
Abstract
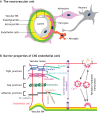
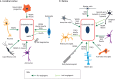
Neurovascular pathologies of the central nervous system (CNS), which are associated with barrier dysfunction, are leading causes of death and disability. The roles that neuronal and glial progenitors and mature cells play in CNS angiogenesis and neurovascular barrier maturation have been elucidated in recent years. Yet how neuronal activity influences these processes remains largely unexplored. Here, we discuss our current understanding of how neuronal and glial development affects CNS angiogenesis and barriergenesis, and outline future directions to elucidate how neuronal activity might influence these processes. An understanding of these mechanisms is crucial for developing new interventions to treat neurovascular pathologies.
Keywords: Angiogenesis; Astrocytes; Basement membrane; Blood-brain barrier; Blood-retina barrier; Light response; Müller glia; Neuroglial progenitors; Neuronal activity; Neurovascular unit; Radial glia; Retinal waves.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare that they have no relevant affiliation or competing financial interests with any organization or entity having a financial interest or conflict with materials discussed in the manuscript.
Figures


References
-
- Arnold T. D., Ferrero G. M., Qiu H., Phan I. T., Akhurst R. J., Huang E. J. and Reichardt L. F. (2012). Defective retinal vascular endothelial cell development as a consequence of impaired integrin alphaVbeta8-mediated activation of transforming growth factor-beta. J. Neurosci. 32, 1197-1206. 10.1523/JNEUROSCI.5648-11.2012 - DOI - PMC - PubMed
-
- Arnold T. D., Niaudet C., Pang M.-F., Siegenthaler J., Gaengel K., Jung B., Ferrero G. M., Mukouyama Y. S., Fuxe J., Akhurst R. et al. (2014). Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking alphaVbeta8-TGFbeta signaling in the brain. Development 141, 4489-4499. 10.1242/dev.107193 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

