Regulatory T cells in skin injury: At the crossroads of tolerance and tissue repair
- PMID: 32358172
- PMCID: PMC7274208
- DOI: 10.1126/sciimmunol.aaz9631
Regulatory T cells in skin injury: At the crossroads of tolerance and tissue repair
Abstract
Skin injury is a highly inflammatory process that is carefully regulated to mitigate tissue damage and allow for proper barrier repair. Regulatory T cells (Tregs) are crucial coordinators of the immune response to injury in several organs. Here, we review the emerging role of Tregs in facilitating skin repair after injury. We focus on recently discovered interactions between lymphocytes and nonhematopoietic cells during wound healing and discuss how these interactions are regulated both by "classical" suppressive mechanisms of Tregs and by "nonclassical" reparative Treg functions.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
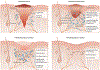


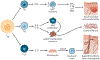
References
-
- Eming SA, Hammerschmidt M, Krieg T & Roers A Interrelation of immunity and tissue repair or regeneration. Semin. Cell Dev. Biol 20, 517–527 (2009). - PubMed

