A novel neurodegenerative spectrum disorder in patients with MLKL deficiency
- PMID: 32358523
- PMCID: PMC7195448
- DOI: 10.1038/s41419-020-2494-0
A novel neurodegenerative spectrum disorder in patients with MLKL deficiency
Abstract
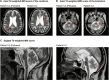
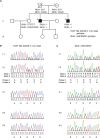
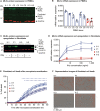
Mixed lineage kinase domain-like (MLKL) is the main executor of necroptosis, an inflammatory form of programmed cell death. Necroptosis is implicated in combating infections, but also in contributing to numerous other clinical conditions, including cardiovascular diseases and neurodegenerative disorders. Inhibition of necroptosis is therefore of therapeutic interest. Here we report two siblings both of whom over the course of 35 years developed a similar progressive, neurodegenerative spectrum disorder characterized by paresis, ataxia and dysarthria. Magnetic resonance imaging of their central nervous system (CNS) revealed severe global cerebral volume loss and atrophy of the cerebellum and brainstem. These brothers are homozygous for a rare haplotype identified by whole genome sequencing carrying a frameshift variant in MLKL, as well as an in-frame deletion of one amino acid in the adjacent fatty acid 2-hydroxylase (FA2H) gene. Functional studies of patient-derived primary cells demonstrated that the variant in MLKL leads to a deficiency of MLKL protein resulting in impairment of necroptosis. Conversely, shotgun lipidomic analysis of the variant in FA2H shows no impact on either the abundance or the enzymatic activity of the encoded hydroxylase. To our knowledge, this is the first report of complete necroptosis deficiency in humans. The findings may suggest that impaired necroptosis is a novel mechanism of neurodegeneration, promoting a disorder that shares some clinical features with primary progressive multiple sclerosis (PPMS) and other neurodegenerative diseases. Importantly, the necroptotic deficiency does not cause symptoms outside the nervous system, nor does it confer susceptibility to infections. Given the current interest in pharmacological inhibition of necroptosis by targeting MLKL and its associated pathways, this strategy should be developed with caution, with careful consideration of the possible development of adverse neurological effects.
Conflict of interest statement
A.B.O. has served on scientific advisory boards for Biogen Idec, Novartis and Sanofi Genzyme; has received research support from Novartis and Biogen Idec; has received speaker honoraria from Biogen Idec, Novartis and TEVA; and has received support for congress participation from, Merck, TEVA, Biogen, Roche, Novartis and Sanofi Genzyme. P.M.M. acknowledges consultancy fees from Adelphi Communications, Biogen, Celgene and Roche; honoraria or speakers’ honoraria from Biogen, Novartis and Roche; research or educational funds from Biogen, GlaxoSmithKline, Nodthera and Novartis; and he is a paid member of the scientific advisory board for Ipsen Pharmaceuticals. None of the companies had any influence over the current work. The other authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- MR/N026934/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_17114/MRC_/Medical Research Council/United Kingdom
- 204290/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- 100308/Z/12/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_12010/3/MRC_/Medical Research Council/United Kingdom
- MC_UU_00008/3/MRC_/Medical Research Council/United Kingdom
- MR/K501013/1/MRC_/Medical Research Council/United Kingdom
- G9901399/MRC_/Medical Research Council/United Kingdom
- G9409531/MRC_/Medical Research Council/United Kingdom
- G0900897/MRC_/Medical Research Council/United Kingdom
- G9409634/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

