Structural changes in noble metal nanoparticles during CO oxidation and their impact on catalyst activity
- PMID: 32358583
- PMCID: PMC7195460
- DOI: 10.1038/s41467-020-16027-9
Structural changes in noble metal nanoparticles during CO oxidation and their impact on catalyst activity
Abstract
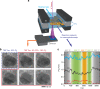
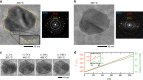
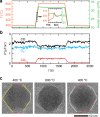
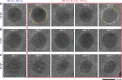
The dynamical structure of a catalyst determines the availability of active sites on its surface. However, how nanoparticle (NP) catalysts re-structure under reaction conditions and how these changes associate with catalytic activity remains poorly understood. Using operando transmission electron microscopy, we show that Pd NPs exhibit reversible structural and activity changes during heating and cooling in mixed gas environments containing O2 and CO. Below 400 °C, the NPs form flat low index facets and are inactive towards CO oxidation. Above 400 °C, the NPs become rounder, and conversion of CO to CO2 increases significantly. This behavior reverses when the temperature is later reduced. Pt and Rh NPs under similar conditions do not exhibit such reversible transformations. We propose that adsorbed CO molecules suppress the activity of Pd NPs at lower temperatures by stabilizing low index facets and reducing the number of active sites. This hypothesis is supported by thermodynamic calculations.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Gustafson J, et al. The role of oxides in catalytic CO oxidation over rhodium and palladium. ACS Catal. 2018;8:4438–4445. doi: 10.1021/acscatal.8b00498. - DOI
-
- Grunwaldt JD, Wagner JB, Dunin-Borkowski RE. Imaging catalysts at work: a hierarchical approach from the macro- to the meso- and nano-scale. ChemCatChem. 2013;5:62–80. doi: 10.1002/cctc.201200356. - DOI
-
- Alayoglu S, Somorjai GA. Nanocatalysis II: in situ surface probes of nano-catalysts and correlative structure–reactivity. Stud. Catal. Lett. 2015;145:249–271. doi: 10.1007/s10562-014-1398-y. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

