Mitochondrial lactate metabolism: history and implications for exercise and disease
- PMID: 32358865
- PMCID: PMC8439166
- DOI: 10.1113/JP278930
Mitochondrial lactate metabolism: history and implications for exercise and disease
Abstract
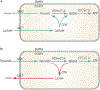
Mitochondrial structures were probably observed microscopically in the 1840s, but the idea of oxidative phosphorylation (OXPHOS) within mitochondria did not appear until the 1930s. The foundation for research into energetics arose from Meyerhof's experiments on oxidation of lactate in isolated muscles recovering from electrical contractions in an O2 atmosphere. Today, we know that mitochondria are actually reticula and that the energy released from electron pairs being passed along the electron transport chain from NADH to O2 generates a membrane potential and pH gradient of protons that can enter the molecular machine of ATP synthase to resynthesize ATP. Lactate stands at the crossroads of glycolytic and oxidative energy metabolism. Based on reported research and our own modelling in silico, we contend that lactate is not directly oxidized in the mitochondrial matrix. Instead, the interim glycolytic products (pyruvate and NADH) are held in cytosolic equilibrium with the products of the lactate dehydrogenase (LDH) reaction and the intermediates of the malate-aspartate and glycerol 3-phosphate shuttles. This equilibrium supplies the glycolytic products to the mitochondrial matrix for OXPHOS. LDH in the mitochondrial matrix is not compatible with the cytoplasmic/matrix redox gradient; its presence would drain matrix reducing power and substantially dissipate the proton motive force. OXPHOS requires O2 as the final electron acceptor, but O2 supply is sufficient in most situations, including exercise and often acute illness. Recent studies suggest that atmospheric normoxia may constitute a cellular hyperoxia in mitochondrial disease. As research proceeds appropriate oxygenation levels should be carefully considered.
Keywords: NADH shuttles; dysoxia; glycolysis; hypoxia; lactic acid; mitochondria; modeling in silico; oxidative phosphorylation; oxygen.
© 2020 The Authors. The Journal of Physiology © 2020 The Physiological Society.
Conflict of interest statement
Figures

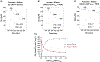
References
-
- DI Abbrescia, La Piana G & Lofrumento NE. (2012). Malate-aspartate shuttle and exogenous NADH/cytochrome c electron transport pathway as two independent cytosolic reducing equivalent transfer systems. Arch Biochem Biophys 518, 157–163. - PubMed
-
- Allchin D. (2002). To err and win a nobel prize: Paul Boyer, ATP synthase and the emergence of bioenergetics. J Hist Biol 35, 149–172. - PubMed
-
- Altinok O, Poggio JL, Stein DE, Bowne WB, Shieh AC, Snyder NW & Orynbayeva Z. (2020). Malate-aspartate shuttle promotes l-lactate oxidation in mitochondria. J Cell Physiol 235, 2569–2581. - PubMed
-
- Altmann R. (1890). Die Elementarorganismen und ihre Beziehungen zu den Zellen. Veit, Leipzig.
-
- Andres R, Cader G & Zierler KL. (1956). The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest 35, 671–682. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

