Vorinostat in the acute neuroinflammatory form of X-linked adrenoleukodystrophy
- PMID: 32359032
- PMCID: PMC7261758
- DOI: 10.1002/acn3.51015
Vorinostat in the acute neuroinflammatory form of X-linked adrenoleukodystrophy
Abstract
Objective: To identify a pharmacological compound targeting macrophages, the most affected immune cells in inflammatory X-linked adrenoleukodystrophy (cerebral X-ALD) caused by ABCD1 mutations and involved in the success of hematopoietic stem cell transplantation and gene therapy.
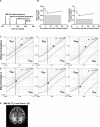
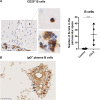
Methods: A comparative database analysis elucidated the epigenetic repressing mechanism of the related ABCD2 gene in macrophages and identified the histone deacetylase (HDAC) inhibitor Vorinostat as a compound to induce ABCD2 in these cells to compensate for ABCD1 deficiency. In these cells, we investigated ABCD2 and pro-inflammatory gene expression, restoration of defective peroxisomal β-oxidation activity, accumulation of very long-chain fatty acids (VLCFAs) and their differentiation status. We investigated ABCD2 and pro-inflammatory gene expression, restoration of defective peroxisomal ß-oxidation activity, accumulation of very long-chain fatty acids (VLCFA) and differentiation status. Three advanced cerebral X-ALD patients received Vorinostat and CSF and MRI diagnostics was carried out in one patient after 80 days of treatment.
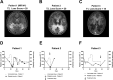
Results: Vorinostat improved the metabolic defects in X-ALD macrophages by stimulating ABCD2 expression, peroxisomal ß-oxidation, and ameliorating VLCFA accumulation. Vorinostat interfered with pro-inflammatory skewing of X-ALD macrophages by correcting IL12B expression and further reducing monocyte differentiation. Vorinostat normalized the albumin and immunoglobulin CSF-serum ratios, but not gadolinium enhancement upon 80 days of treatment.
Interpretation: The beneficial effects of HDAC inhibitors on macrophages in X-ALD and the improvement of the blood-CSF/blood-brain barrier are encouraging for future investigations. In contrast with Vorinostat, less toxic macrophage-specific HDAC inhibitors might improve also the clinical state of X-ALD patients with advanced inflammatory demyelination.
© 2020 The Authors. Annals of Clinical and Translational Neurology published by Wiley Periodicals, Inc on behalf of American Neurological Association.
Conflict of interest statement
We declare that we have no competing interests.
Figures




References
-
- Moser HW, Smith KD, Watkins PA, et al. X‐linked Adrenoleukodystrophy In: Scriver AL, Beaudet AL, Sly WS. and Valle DCR, eds. The metabolic and molecular bases of inherited disease. pp. 3257–3301. New York: McGraw Hill, 2001.
-
- Mosser J, Douar AM, Sarde CO, et al. Putative X‐linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 1993;361:726–730. - PubMed
-
- Berger J, Gartner J. X‐linked adrenoleukodystrophy: clinical, biochemical and pathogenetic aspects. Biochim Biophys Acta 2006;1763:1721–1732. - PubMed
-
- Kemp S, Berger J, Aubourg P. X‐linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim Biophys Acta (BBA)‐Mol Basis Dis 2012;1822:1465–1474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

