A Rapid Systematic Review of Clinical Trials Utilizing Chloroquine and Hydroxychloroquine as a Treatment for COVID-19
- PMID: 32359203
- PMCID: PMC7267507
- DOI: 10.1111/acem.14005
A Rapid Systematic Review of Clinical Trials Utilizing Chloroquine and Hydroxychloroquine as a Treatment for COVID-19
Abstract
Objectives: The emergence of SARS-CoV-2 has presented clinicians with a difficult therapeutic dilemma. With supportive care as the current mainstay of treatment, the fatality rate of COVID-19 is 6.9%. There are currently several trials assessing the efficacy of different antivirals as treatment. Of these, chloroquine (CQ) and its derivative hydroxychloroquine (HCQ) have garnered the most attention.
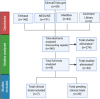
Methods: In this study, the literature currently available on CQ and HCQ as treatment of COVID-19 was surveyed using EMBASE, PubMed, Cochrane Library, MedRxiv, and one clinical trial registry. Upon gathering published and preprint trials, risk of bias was assessed using Cochrane Risk of Bias Tool 2.0.
Results: There are currently seven completed clinical trials and 29 registered clinical trials focusing on HCQ or CQ as a therapeutic avenue for COVID-19. Of these, five of seven trials have shown favorable outcomes for patients using CQ or HCQ and two of seven have shown no change compared to control. However, all seven trials carried varying degrees of bias and poor study design.
Conclusion: There are currently not enough data available to support the routine use of HCQ and CQ as therapies for COVID-19. Pending further results from more extensive studies with more stringent study parameters, clinicians should defer from routine use of HCQ and CQ. There are several clinical trials currently under way with results expected soon.
© 2020 by the Society for Academic Emergency Medicine.
Figures

References
-
- WHO Director‐General's Opening Remarks at the Media Briefing on COVID‐19 – 11 March 2020. World Health Organization. Available at: https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐re.... Accessed Mar 11, 2020.
-
- Disposition of Non‐Hospitalized Patients with COVID‐19. Centers for Disease Control and Prevention. Available at: www.cdc.gov/coronavirus/2019‐ncov/hcp/disposition‐in‐home‐patients.html. Accessed Apr 10, 2020.
-
- Return‐to‐Work Criteria for Healthcare Workers . Centers for Disease Control and Prevention. Available at: www.cdc.gov/coronavirus/2019‐ncov/hcp/return‐to‐work.html?CDC_AA_refVal=.... Accessed Apr 13, 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

