A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice
- PMID: 32359470
- PMCID: PMC7335735
- DOI: 10.1016/j.ymthe.2020.04.018
A Multi-Targeting, Nucleoside-Modified mRNA Influenza Virus Vaccine Provides Broad Protection in Mice
Abstract
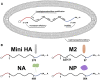
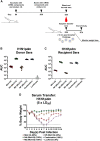
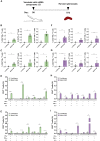
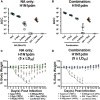
Influenza viruses are respiratory pathogens of public health concern worldwide with up to 650,000 deaths occurring each year. Seasonal influenza virus vaccines are employed to prevent disease, but with limited effectiveness. Development of a universal influenza virus vaccine with the potential to elicit long-lasting, broadly cross-reactive immune responses is necessary for reducing influenza virus prevalence. In this study, we have utilized lipid nanoparticle-encapsulated, nucleoside-modified mRNA vaccines to intradermally deliver a combination of conserved influenza virus antigens (hemagglutinin stalk, neuraminidase, matrix-2 ion channel, and nucleoprotein) and induce strong immune responses with substantial breadth and potency in a murine model. The immunity conferred by nucleoside-modified mRNA-lipid nanoparticle vaccines provided protection from challenge with pandemic H1N1 virus at 500 times the median lethal dose after administration of a single immunization, and the combination vaccine protected from morbidity at a dose of 50 ng per antigen. The broad protective potential of a single dose of combination vaccine was confirmed by challenge with a panel of group 1 influenza A viruses. These findings support the advancement of nucleoside-modified mRNA-lipid nanoparticle vaccines expressing multiple conserved antigens as universal influenza virus vaccine candidates.
Keywords: influenza virus; lipid nanoparticle; mRNA; nucleoside-modification; universal vaccine; vaccine.
Copyright © 2020 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Preparing for Pandemics: RNA Vaccines at the Forefront.Mol Ther. 2020 Jul 8;28(7):1559-1560. doi: 10.1016/j.ymthe.2020.06.017. Epub 2020 Jun 23. Mol Ther. 2020. PMID: 32579882 Free PMC article. No abstract available.
References
-
- World Health Organization . 2020. Influenza (seasonal) fact sheet.https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal
-
- Centers for Disease Control and Prevention . 2019. Seasonal influenza vaccine effectiveness, 2004–2019.https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm
-
- Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:36–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

