Cognitive impairment appears progressive in the mdx mouse
- PMID: 32360405
- PMCID: PMC7306157
- DOI: 10.1016/j.nmd.2020.02.018
Cognitive impairment appears progressive in the mdx mouse
Abstract
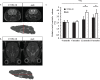
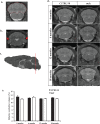
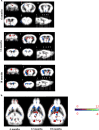
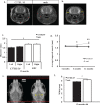
Duchenne muscular dystrophy (DMD) is an X-linked recessive muscle wasting disease caused by mutations in the DMD gene, which encodes the large cytoskeletal protein dystrophin. Approximately one-third of DMD patient's exhibit cognitive problems yet it is unknown if cognitive impairments worsen with age. The mdx mouse model is deficient in dystrophin demonstrates cognitive abnormalities, but no studies have investigated this longitudinally. We assessed the consequences of dystrophin deficiency on brain morphology and cognition in male mdx mice. We utilised non-invasive methods to monitor CNS pathology with an aim to identify changes longitudinally (between 4 and 18 months old) which could be used as outcome measures. MRI identified a total brain volume (TBV) increase in control mice with ageing (p < 0.05); but the mdx mice TBV increased significantly more (p < 0.01). Voxel-based morphometry (VBM) identified decreases in grey matter volume, particularly in the hippocampus of the mdx brain, most noticeable from 12 months onwards, as were enlarged lateral ventricles in mdx mice. The caudate putamen of older mdx mice showed increases in T2- relaxometry which may be considered as evidence of increased water content. Hippocampal spatial learning and memory was decreased in mdx mice, particularly long-term memory, which progressively worsened with age. The novel object recognition (NOR) task highlighted elevated anxiety-related behaviour in older mdx mice. Our studies suggest that dystrophin deficiency causes a progressive cognitive impairment in mice (compared to ageing control mice), becoming evident at late disease stages, and may explain why progressive CNS symptoms are not obvious in DMD patients.
Keywords: Cognitive behaviour; Duchenne muscular dystrophy (DMD); Magnetic resonance imaging (MRI); Mdx mouse.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Figures






References
-
- Emery A.E. Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord. 1991;1(1):19–29. - PubMed
-
- Mah J.K., Korngut L., Dykeman J., Day L., Pringsheim T., Jette N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul Disord. 2014;24(6):482–491. - PubMed
-
- Wallace G.Q., McNally E.M. Mechanisms of muscle degeneration, regeneration, and repair in the muscular dystrophies. Annu Rev Physiol. 2009;71:37–57. - PubMed
-
- Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51(6):919–928. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

