Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features
- PMID: 32360716
- PMCID: PMC7492441
- DOI: 10.1016/j.jaad.2020.04.105
Dermatologic toxicities to immune checkpoint inhibitor therapy: A review of histopathologic features
Abstract
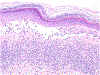
Antineoplastic agents that use the immune system have revolutionized cancer treatment. Specifically, implementation of immune checkpoint inhibitors, monoclonal antibodies that block cytotoxic T-lymphocyte-associated antigen-4, programmed cell death protein 1, or programmed cell death ligand 1 show improved and sustained responses in patients with cancer. However, these agents are associated with a plethora of adverse events, many manifesting in the skin. As the clinical application of cancer immunotherapies expands, understanding the clinical and histopathologic features of associated cutaneous toxicities becomes increasingly important to dermatologists, oncologists, and pathologists to ensure timely diagnosis and appropriate care. This review discusses cutaneous reactions to immune checkpoint inhibitors, focusing on histopathologic features.
Keywords: CTLA-4; PD-L1; PD1; adverse event; atezolizumab; avelumab; bullous pemphigoid; checkpoint inhibitor; cutaneous; durvalumab; immunotherapy; ipilimumab; lichenoid dermatitis; nivolumab; pembrolizumab; rash; skin; toxicity.
Copyright © 2020 American Academy of Dermatology, Inc. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature reviews Immunology 2008;8:467–77. - PubMed
-
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–9. - PubMed

