Reductive stress impairs myogenic differentiation
- PMID: 32361680
- PMCID: PMC7199008
- DOI: 10.1016/j.redox.2020.101492
Reductive stress impairs myogenic differentiation
Abstract
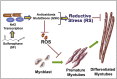
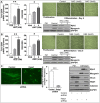
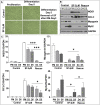
Myo-satellite cells regenerate and differentiate into skeletal muscle (SM) after acute or chronic injury. Changes in the redox milieu towards the oxidative arm at the wound site are known to compromise SM regeneration. Recently, we reported that abrogation of Nrf2/antioxidant signaling promotes oxidative stress and impairs SM regeneration in C57/Bl6 mice. Here, we investigated whether the activation of intracellular Nrf2 signaling favors antioxidant transcription and promotes myoblast differentiation. Satellite cell-like C2C12 myoblasts were treated with sulforaphane (SF; 1.0 & 5.0 μM) to activate Nrf2/antioxidant signaling during proliferation and differentiation (i.e. formation of myotubes/myofibers). SF-mediated Nrf2 activation resulted in increased expression of Nrf2-antioxidants (e.g. GCLC and G6PD) and augmented the production of reduced glutathione (GSH) leading to a reductive redox state. Surprisingly, this resulted in significant inhibition of myoblast differentiation, as observed from morphological changes and reduced expression of MyoD, Pax7, and Myh2, due to reductive stress (RS). Furthermore, supplementation of N-acetyl-cysteine (NAC) or GSH-ester or genetic knock-down of Keap1 (using siRNA) also resulted in RS-driven inhibition of differentiation. Interestingly, withdrawing Nrf2 activation rescued differentiation potential and formation of myotubes/myofibers from C2C12 myoblasts. Thus, abrogation of physiological ROS signaling through over-activation of Nrf2 (i.e. RS) and developing RS hampers differentiation of muscle satellite cells.
Keywords: Differentiation markers; Nrf2-signaling; Pro-oxidative setting; Reactive oxygen species (ROS); Reductive stress; Satellite cells; Skeletal muscle regeneration.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no conflict of interests.
Figures


References
-
- Bo H. Mitochondrial redox metabolism in aging: effect of exercise interventions. J. Sport Health Sci. 2013;2(2):67–74.
-
- Agerholm M. Perturbations of NAD(+) salvage systems impact mitochondrial function and energy homeostasis in mouse myoblasts and intact skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2018;314(4):E377–e395. - PubMed
-
- Ehrhardt J., Morgan J. Regenerative capacity of skeletal muscle. Curr. Opin. Neurol. 2005;18(5):548–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

