Advances in Clinical Cardiology 2019: A Summary of Key Clinical Trials
- PMID: 32361851
- PMCID: PMC7467440
- DOI: 10.1007/s12325-020-01355-5
Advances in Clinical Cardiology 2019: A Summary of Key Clinical Trials
Abstract
Introduction: A large number of important clinical trials in cardiology were published or presented at major international conferences during 2019. This paper aims to offer a concise overview of these significant advances and to put them into clinical context.
Methods: Trials presented at the major international cardiology meetings during 2019 were reviewed including The American College of Cardiology (ACC), Euro PCR, The European Society of Cardiology (ESC), Transcatheter Cardiovascular Therapeutics (TCT), and the American Heart Association (AHA). In addition to this a literature search identified several other publications eligible for inclusion based on their relevance to clinical cardiology, their potential impact on clinical practice and on future guidelines.
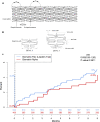
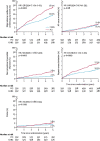
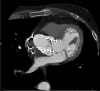
Results: A total of 70 trials met the inclusion criteria. New interventional and structural data include trials examining use of drug-coated balloons in patients with acute myocardial infarction (MI), interventions following shockable cardiac arrest, mechanical circulatory support in cardiogenic shock complicating MI, intervention in stable coronary artery disease, surgical or percutaneous revascularisation strategies in left main coronary artery disease, revascularisation strategy in ST elevation MI, transcatheter aortic valve replacement in low-risk patients, and percutaneous mitral or tricuspid valve interventions. Preventative cardiology data included the use of sodium-glucose cotransporter 2 (SGLT2) inhibitors (dapagliflozin), proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors (evolocumab), bempedoic acid, and novel approaches to the management of hypertension. Antiplatelet data included trials evaluating both the optimal length of course and combination of antiplatelet agents and regimes including combination antithrombotic therapies for patients with atrial fibrillation. Heart failure data included trials of sacubitril-valsartan in heart failure with preserved ejection fraction and the use of SGLT2 inhibitors in patients with heart failure but without diabetes. Electrophysiology data included trials examining alcohol in atrial fibrillation and the use of wearable fitness devices for identifying atrial fibrillation.
Conclusion: This article presents key clinical trials completed during 2019 and should be valuable to clinicians and researchers working in cardiology.
Keywords: Acute coronary syndrome; Anticoagulation; Atrial fibrillation; Cardiology; Coronary revascularisation; Heart failure; Lipids; Mitral clip; Myocardial infarction; Transcatheter aortic valve implantation.
Conflict of interest statement
Katie Linden, Jonathan Mailey and Aileen Kearney have nothing to disclose. Ian B. A. Menown has received grants to institution, honoraria and/or conference sponsorship from Biosensors, Boston Scientific, Meril Life, Orbus Neich, Astra Zeneca, Amgen, Bayer, Boehringer Ingelheim, Daichii Sankyo, Lilly, Bristol Myers Squibb, Pfizer, and Sanofi Aventis. Ian Menown is the Editor-in-Chief of this journal.
Figures


Similar articles
-
Advances in Clinical Cardiology 2022: A Summary of Key Clinical Trials.Adv Ther. 2023 Jun;40(6):2595-2625. doi: 10.1007/s12325-023-02502-4. Epub 2023 Apr 13. Adv Ther. 2023. PMID: 37052800 Free PMC article. Review.
-
Advances in Clinical Cardiology 2018: A Summary of Key Clinical Trials.Adv Ther. 2019 Jul;36(7):1549-1573. doi: 10.1007/s12325-019-00962-1. Epub 2019 May 7. Adv Ther. 2019. PMID: 31065993 Free PMC article. Review.
-
Advances in Clinical Cardiology 2021: A Summary of Key Clinical Trials.Adv Ther. 2022 Jun;39(6):2398-2437. doi: 10.1007/s12325-022-02136-y. Epub 2022 Apr 28. Adv Ther. 2022. PMID: 35482250 Free PMC article. Review.
-
Advances in Clinical Cardiology 2020: A Summary of Key Clinical Trials.Adv Ther. 2021 May;38(5):2170-2200. doi: 10.1007/s12325-021-01711-z. Epub 2021 Apr 12. Adv Ther. 2021. PMID: 33844133 Free PMC article. Review.
-
Advances in Clinical Cardiology 2017: A Summary of Key Clinical Trials.Adv Ther. 2018 Jul;35(7):899-927. doi: 10.1007/s12325-018-0716-y. Epub 2018 Jun 14. Adv Ther. 2018. PMID: 29949039 Free PMC article. Review.
Cited by
-
SGLT2 Inhibitors and Improved Survival in Patients with Diabetes and Acute Myocardial Infarction: Evidence from an Electronic Health Record-Based Cohort Study.Am J Cardiovasc Drugs. 2025 Aug 21. doi: 10.1007/s40256-025-00759-4. Online ahead of print. Am J Cardiovasc Drugs. 2025. PMID: 40839161
-
Advances in Clinical Cardiology 2022: A Summary of Key Clinical Trials.Adv Ther. 2023 Jun;40(6):2595-2625. doi: 10.1007/s12325-023-02502-4. Epub 2023 Apr 13. Adv Ther. 2023. PMID: 37052800 Free PMC article. Review.
-
Advances in Clinical Cardiology 2024: A Summary of Key Clinical Trials.Adv Ther. 2025 Jul;42(7):3111-3140. doi: 10.1007/s12325-025-03220-9. Epub 2025 May 19. Adv Ther. 2025. PMID: 40388090 Free PMC article. Review.
-
Role of Vasoactive Hormone-Induced Signal Transduction in Cardiac Hypertrophy and Heart Failure.Cells. 2024 May 17;13(10):856. doi: 10.3390/cells13100856. Cells. 2024. PMID: 38786079 Free PMC article. Review.
-
Advances in Clinical Cardiology 2023: A Summary of Key Clinical Trials.Adv Ther. 2024 Jul;41(7):2606-2634. doi: 10.1007/s12325-024-02877-y. Epub 2024 May 14. Adv Ther. 2024. PMID: 38743242 Free PMC article. Review.
References
-
- Zaman A, de Winter RJ, Kogame N, et al. Safety and efficacy of a sirolimus-eluting coronary stent with ultra-thin strut for treatment of atherosclerotic lesions (TALENT): a prospective multicentre randomised controlled trial. Lancet. 2019;393(10175):987–997. doi: 10.1016/S0140-6736(18)32467-X. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

