Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering
- PMID: 32361917
- PMCID: PMC7311562
- DOI: 10.1007/s10815-020-01784-9
Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering
Erratum in
-
Correction to: Whole-ovary decellularization generates an effective 3D bioscaffold for ovarian bioengineering.J Assist Reprod Genet. 2023 Sep;40(9):2279. doi: 10.1007/s10815-023-02894-w. J Assist Reprod Genet. 2023. PMID: 37501007 Free PMC article. No abstract available.
Abstract
Purpose: To develop a new protocol for whole-ovary decellularization for the production of a 3D bioscaffold suitable for in vitro/ex vivo studies and for the reconstruction of a bioengineered ovary.
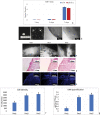
Methods: Porcine ovaries were subjected to the decellularization process (DECELL; n = 20) that involved a freeze-thaw cycle, followed by sequential incubations in 0.5% SDS for 3 h, 1% Triton X-100 for 9 h, and 2% deoxycholate for 12 h. Untreated ovaries were used as a control (CTR; n = 6). Both groups were analyzed to evaluate cell and DNA removal as well as ECM preservation. DECELL bioscaffolds were assessed for cytotoxicity and cell homing ability.
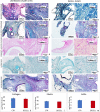
Results: DECELL ovaries maintained shape and homogeneity without any deformation, while their color turned from red to white. Histological staining and DNA quantification confirmed a decrease of 98.11% in DNA content, compared with the native tissue (CTR). Histochemical assessments demonstrated the preservation of intact ECM microarchitecture after the decellularization process. This was also confirmed by quantitative analysis of collagen, elastin, and GAG contents. DECELL bioscaffold showed no cytotoxic effects in co-culture and, when re-seeded with homologous fibroblasts, encouraged a rapid cell adhesion and migration, with repopulating cells increasing in number and aggregating in cluster-like structures, consistent with its ability to sustain cell adherence, proliferation, and differentiation.
Conclusion: The protocol described allows for the generation of a 3D bioscaffold that may constitute a suitable model for ex vivo culture of ovarian cells and follicles, as well as a promising tool for the reconstruction of a bioengineered ovary.
Keywords: 3D bioscaffold; Decellularization; Extracellular matrix; Porcine; Whole ovary.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Trinh X-B, Peeters F, Tjalma WAA. The thoughts of breast cancer survivors regarding the need for starting hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol. 2006;124:250–253. - PubMed
-
- Yalcinkaya TM, Sittadjody S, Opara EC. Scientific principles of regenerative medicine and their application in the female reproductive system. Maturitas. 2014;77:12–19. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

