Cognitive Efficacy of Pharmacologic Treatments in Multiple Sclerosis: A Systematic Review
- PMID: 32361940
- PMCID: PMC7275014
- DOI: 10.1007/s40263-020-00734-4
Cognitive Efficacy of Pharmacologic Treatments in Multiple Sclerosis: A Systematic Review
Abstract
Introduction: Cognitive impairment is prevalent and debilitating among persons with multiple sclerosis (MS). While many pharmacologic treatments have shown good efficacy in reducing clinical relapses, brain lesions, and improving certain physical symptoms, their efficacy for improving cognitive function is not well understood.
Objectives: The current systematic review aimed to evaluate the efficacy of pharmacologic treatments for improving cognitive function among persons with MS.
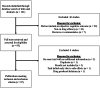
Methods: A literature search was conducted through the PubMed and PsycINFO databases. Two independent reviewers assessed each paper, and a third reviewer weighed in if the two reviewers could not reach a consensus. Classification of evidence was determined using the 2017 American Academy of Neurology (AAN) criteria for therapeutic trials. Standardized effect sizes (Cohen's d) were calculated to compare across studies.
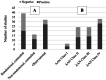
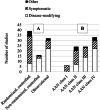
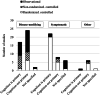
Results: Eighty-seven journal articles published between 1990 and January 2020 were included in the current review. Overall, there is insufficient evidence to support the use of pharmacologic treatments to improve cognitive function in persons with MS. There were many contradictory findings observed in this review, which may be due to possible unidentified moderating treatment response variables and/or lack of standardization in assessment procedures. There was also an overreliance on statistical significance (most papers did not provide sizes of treatment effects), which may not be clinically meaningful.
Conclusions: Higher-quality randomized controlled trials are needed to establish the cognitive efficacy of pharmacologic treatments for MS-related cognitive dysfunction, with cognition as the primary endpoint. Researchers are urged to use standardized criteria (such as the AAN criteria) to guide their research designs. Clinicians should consider effect sizes of studies before deciding whether to prescribe certain medications to ameliorate cognitive symptoms.
Conflict of interest statement
Helen M. Genova has received funding from the National Multiple Sclerosis Society. John DeLuca has received funding from the National Multiple Sclerosis Society; served as a consultant or member of the advisory board for Biogen, Celgene, Novartis, and MedRhythms; provided paid lectures for Biogen and Sanofi-Genzyme; and received book loyalties. Michelle H. Chen and Yael Goverover declare no potential conflicts of interest.
Figures
References
-
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–1151. - PubMed
-
- Langdon D. Cognition in multiple sclerosis. Curr Opin Neurol. 2011;24(3):244–249. - PubMed
-
- Strober LB, Christodoulou C, Benedict RH, Westervelt HJ, Melville P, Scherl WF, et al. Unemployment in multiple sclerosis: the contribution of personality and disease. Mult Scler J. 2012;18(5):647–653. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

