Dynamic network connectivity predicts subjective cognitive decline: the Sino-Longitudinal Cognitive impairment and dementia study
- PMID: 32361946
- PMCID: PMC7606422
- DOI: 10.1007/s11682-019-00220-6
Dynamic network connectivity predicts subjective cognitive decline: the Sino-Longitudinal Cognitive impairment and dementia study
Abstract
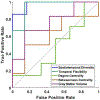
Subjective cognitive decline (SCD) is the preclinical stage of Alzheimer's disease (AD), the most common neurodegenerative disease in the elderly. We collected resting-state functional MRI data and applied novel graph-theoretical analyses to investigate the dynamic spatiotemporal cerebral connectivities in 63 individuals with SCD and 67 normal controls (NC). Temporal flexibility and spatiotemporal diversity were mapped to reflect dynamic time-varying functional interactions among the brain regions within and outside communities. Temporal flexibility indicates how frequently a brain region interacts with regions of other communities across time; spatiotemporal diversity describes how evenly a brain region interacts with regions belonging to other communities. SCD and NC differed in large-scale brain dynamics characterized by the two measures, which, with support vector machine, demonstrated higher classification accuracies than conventional static parameters and structural metrics. The findings characterize dynamic network dysfunction that may serve as a biomarker of the preclinical stage of AD.
Keywords: Alzheimer’s disease; Resting-state functional MRI; Spatiotemporal diversity; Subjective cognitive decline; Temporal flexibility.
Conflict of interest statement
Figures




Similar articles
-
Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer's disease (DELCODE).Alzheimers Res Ther. 2018 Feb 7;10(1):15. doi: 10.1186/s13195-017-0314-2. Alzheimers Res Ther. 2018. PMID: 29415768 Free PMC article.
-
Application of advanced machine learning methods on resting-state fMRI network for identification of mild cognitive impairment and Alzheimer's disease.Brain Imaging Behav. 2016 Sep;10(3):799-817. doi: 10.1007/s11682-015-9448-7. Brain Imaging Behav. 2016. PMID: 26363784
-
Functional Connectivity Dynamics Altered of the Resting Brain in Subjective Cognitive Decline.Front Aging Neurosci. 2022 Jun 24;14:817137. doi: 10.3389/fnagi.2022.817137. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35813944 Free PMC article.
-
Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer's disease.Mol Neurodegener. 2020 Sep 22;15(1):55. doi: 10.1186/s13024-020-00395-3. Mol Neurodegener. 2020. PMID: 32962744 Free PMC article. Review.
-
A survey on applications and analysis methods of functional magnetic resonance imaging for Alzheimer's disease.J Neurosci Methods. 2019 Apr 1;317:121-140. doi: 10.1016/j.jneumeth.2018.12.012. Epub 2018 Dec 26. J Neurosci Methods. 2019. PMID: 30593787 Review.
Cited by
-
Relationship among number of close friends, subclinical geriatric depression, and subjective cognitive decline based on regional homogeneity of functional magnetic resonance imaging data.Front Aging Neurosci. 2022 Sep 23;14:978611. doi: 10.3389/fnagi.2022.978611. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36212042 Free PMC article.
-
Characterizing the progression from mild cognitive impairment to dementia: a network analysis of longitudinal clinical visits.BMC Med Inform Decis Mak. 2024 Oct 18;24(1):305. doi: 10.1186/s12911-024-02711-z. BMC Med Inform Decis Mak. 2024. PMID: 39425117 Free PMC article.
-
Drinking severity mediates the relationship between hypothalamic connectivity and rule-breaking/intrusive behavior differently in young women and men: an exploratory study.Quant Imaging Med Surg. 2024 Sep 1;14(9):6669-6683. doi: 10.21037/qims-24-815. Epub 2024 Aug 26. Quant Imaging Med Surg. 2024. PMID: 39281112 Free PMC article.
-
Grey matter changes on brain MRI in subjective cognitive decline: a systematic review.Alzheimers Res Ther. 2022 Jul 22;14(1):98. doi: 10.1186/s13195-022-01031-6. Alzheimers Res Ther. 2022. PMID: 35869559 Free PMC article.
-
Plasma β-Amyloid Levels Associated With Structural Integrity Based on Diffusion Tensor Imaging in Subjective Cognitive Decline: The SILCODE Study.Front Aging Neurosci. 2021 Jan 12;12:592024. doi: 10.3389/fnagi.2020.592024. eCollection 2020. Front Aging Neurosci. 2021. PMID: 33510631 Free PMC article.
References
MeSH terms
Grants and funding
- R21 DA044749/DA/NIDA NIH HHS/United States
- 81471743/National Natural Science Foundation of China
- UL1 TR001863/TR/NCATS NIH HHS/United States
- 81430037/National Natural Science Foundation of China
- 2016YFC1306300/The National Key Research and Development Program of China
- 81471731/National Natural Science Foundation of China
- 7161009/Beijing Nature Science Foundation
- 61633018/National Natural Science Foundation of China
- PXM2019_026283_000002/Beijing Municipal Commission of Health and Family Planning
- R21DA044749-02S1/NH/NIH HHS/United States
- 81801052/National Natural Science Foundation of China
- R21 AG067024/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

