Chloroplast-associated molecular patterns as concept for fine-tuned operational retrograde signalling
- PMID: 32362264
- PMCID: PMC7209960
- DOI: 10.1098/rstb.2019.0443
Chloroplast-associated molecular patterns as concept for fine-tuned operational retrograde signalling
Abstract
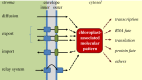
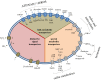
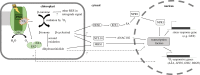
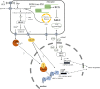
Chloroplasts compose about one-quarter of the mesophyll cell volume and contain about 60% of the cell protein. Photosynthetic carbon assimilation is the dominating metabolism in illuminated leaves. To optimize the resource expenditure in these costly organelles and to control and adjust chloroplast metabolism, an intensive transfer of information between nucleus-cytoplasm and chloroplasts occurs in both directions as anterograde and retrograde signalling. Recent research identified multiple retrograde pathways that use metabolite transfer and include reaction products of lipids and carotenoids with reactive oxygen species (ROS). Other pathways use metabolites of carbon, sulfur and nitrogen metabolism, low molecular weight antioxidants and hormone precursors to carry information between the cell compartments. This review focuses on redox- and ROS-related retrograde signalling pathways. In analogy to the microbe-associated molecular pattern, we propose the term 'chloroplast-associated molecular pattern' which connects chloroplast performance to extrachloroplast processes such as nuclear gene transcription, posttranscriptional processing, including translation, and RNA and protein fate. This article is part of the theme issue 'Retrograde signalling from endosymbiotic organelles'.
Keywords: hormone; oxylipin; photosynthesis; reactive oxygen species; redox regulation; retrograde signalling.
Conflict of interest statement
The authors do not encounter any conflict of interest.
Figures
References
-
- Dietz KJ, Busch H. 1990. Compartmentation, utilisation and transport of methionine in barley mesophyll cells. In Sulfur nutrition and sulfur assimilation in higher plants (eds Rennenberg H, Brunold CH, De Kok LJ, Stulen I), pp. 177–180. The Hague, The Netherlands: SPB Academic Publishing.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
