Molecular Basis for Synaptotagmin-1-Associated Neurodevelopmental Disorder
- PMID: 32362337
- PMCID: PMC7539681
- DOI: 10.1016/j.neuron.2020.04.003
Molecular Basis for Synaptotagmin-1-Associated Neurodevelopmental Disorder
Abstract
At neuronal synapses, synaptotagmin-1 (syt1) acts as a Ca2+ sensor that synchronizes neurotransmitter release with Ca2+ influx during action potential firing. Heterozygous missense mutations in syt1 have recently been associated with a severe but heterogeneous developmental syndrome, termed syt1-associated neurodevelopmental disorder. Well-defined pathogenic mechanisms, and the basis for phenotypic heterogeneity in this disorder, remain unknown. Here, we report the clinical, physiological, and biophysical characterization of three syt1 mutations from human patients. Synaptic transmission was impaired in neurons expressing mutant variants, which demonstrated potent, graded dominant-negative effects. Biophysical interrogation of the mutant variants revealed novel mechanistic features concerning the cooperative action, and functional specialization, of the tandem Ca2+-sensing domains of syt1. These mechanistic studies led to the discovery that a clinically approved K+ channel antagonist is able to rescue the dominant-negative heterozygous phenotype. Our results establish a molecular cause, basis for phenotypic heterogeneity, and potential treatment approach for syt1-associated neurodevelopmental disorder.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
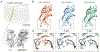
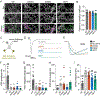




References
-
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH, 2010. PHENIX : a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

