Effect of Dalbavancin on Staphylococcal Biofilms When Administered Alone or in Combination With Biofilm-Detaching Compounds
- PMID: 32362877
- PMCID: PMC7180179
- DOI: 10.3389/fmicb.2020.00553
Effect of Dalbavancin on Staphylococcal Biofilms When Administered Alone or in Combination With Biofilm-Detaching Compounds
Abstract
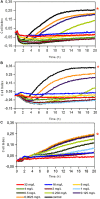
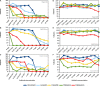
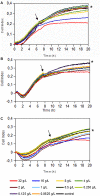
Microorganisms grown in biofilms are more resistant to antimicrobial treatment and immune system attacks compared to their planktonic forms. In fact, infections caused by biofilm-forming Staphylococcus aureus and Staphylococcus epidermidis are a large threat for public health, including patients with medical devices. The aim of the current manuscript was to test the effect of dalbavancin, a recently developed lipoglycopeptide antibiotic, alone or in combination with compounds contributing to bacterial cell disaggregation, on staphylococcal biofilm formation and elimination. We used real-time impedance measurements in microtiter plates to study biofilm growth dynamics of S. aureus and S. epidermidis strains, in the absence or presence of dalbavancin, linezolid, vancomycin, cloxacillin, and rifampicin. Further experiments were undertaken to check whether biofilm-detaching compounds such as N-acetylcysteine (NAC) and ficin could enhance dalbavancin efficiency. Real-time dose-response experiments showed that dalbavancin is a highly effective antimicrobial, preventing staphylococcal biofilm formation at low concentrations. Minimum biofilm inhibitory concentrations were up to 22 higher compared to standard E-test values. Dalbavancin was the only antimicrobial that could halt new biofilm formation on established biofilms compared to the other four antibiotics. The addition of NAC decreased dalbavancin efficacy while the combination of dalbavancin with ficin was more efficient than antibiotic alone in preventing growth once the biofilm was established. Results were confirmed by classical biofilm quantification methods such as crystal violet (CV) staining and viable colony counting. Thus, our data support the use of dalbavancin as a promising antimicrobial to treat biofilm-related infections. Our data also highlight that synergistic and antagonistic effects between antibiotics and biofilm-detaching compounds should be carefully tested in order to achieve an efficient treatment that could prevent both biofilm formation and disruption.
Keywords: E-test; N-acetylcysteine; Staphylococcus; biofilm; dalbavancin; ficin; xCELLigence.
Copyright © 2020 Žiemytė, Rodríguez-Díaz, Ventero, Mira and Ferrer.
Figures



References
-
- Baldoni D., Furustrand Tafin U., Aeppli S., Angevaare E., Oliva A., Haschke M., et al. (2013). Activity of dalbavancin, alone and in combination with rifampicin, against meticillin-resistant Staphylococcus aureus in a foreign-body infection model. Int. J. Antimicrob. Agents 42 220–225. 10.1016/j.ijantimicag.2013.05.019 - DOI - PubMed

