Use of a Neonatal-Mouse Model to Characterize Vaccines and Strategies for Overcoming the High Susceptibility and Severity of Pertussis in Early Life
- PMID: 32362890
- PMCID: PMC7182080
- DOI: 10.3389/fmicb.2020.00723
Use of a Neonatal-Mouse Model to Characterize Vaccines and Strategies for Overcoming the High Susceptibility and Severity of Pertussis in Early Life
Abstract
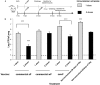
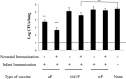
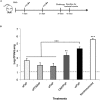
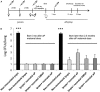
Newborns and unvaccinated infants, compared to other age groups, are more susceptible to pertussis infection, manifesting severe symptoms leading to a higher mortality. The recent increase in pertussis cases demands more effective strategies to overcome this major health problem. In parallel with maternal-immunization, neonatal-immunization (NI) is a strategy needing revision. Here, using the intranasal-challenge-mouse-model we evaluated the protective capacity of NI in both naïve-mice and those with maternally acquired immunity. We tested our acellular-vaccine-candidate based on outer-membrane-vesicles derived from Bordetella pertussis (OMVP) that induces Th2-profile but also the recommended Th-profile for protection: Th1/Th17-profile and CD4 T-memory-cells that reside in the lungs. Commercial acellular-vaccine (aP) and whole cell-vaccine (wP) inducing mainly Th2-profile and Th1-profile, respectively, were also tested. Analyzing the induced immunity and protection capability of NI included in 1- or 2-dose schedules with the same or different types of vaccine, we detected that the aP-vaccine administered in either single- or 2-dose schedules protected against sublethal B. pertussis infection. Schedules consisting of doses of aP neonatally and of OMVP or wP vaccine during infancy greatly reduced bacterial lung colonization while inducing the highest levels of high-avidity anti-pertussis toxin (PTx) IgG. That OMVP or wP neonatal dose did not interfere with the protection of transferred maternal immunity was especially encouraging. Moreover, OMVP- or wP used as a neonatal dose enhanced the quality of the humoral immune response in immunized pups. Antibodies generated by OMVP-or wP-vaccinated mice born to aP-immunized mothers were of higher avidity than those from mice that harbored only maternal immunity; but when mothers and neonates were immunized with the same aP-vaccine, the humoral response in the neonates was partially suppressed through the blunting of the level of anti-PTx IgG induced by the neonatal aP dose. These results demonstrated that neonatal immunization is a possible strategy to be considered to improve the current pertussis epidemiology. For neonates without maternal-immunity, mixed-vaccination schedules that include the aP- and OMVP-vaccines appear to be the most appropriate to induce protection in the pups. For offspring from immune mothers, to avoid blunting-effect, NI should be carried out with vaccines other than those applied during pregnancy.
Keywords: Bordetella pertussis; neonatal immunization; outer-membrane vesicles; pertussis; protection.
Copyright © 2020 Martin Aispuro, Ambrosis, Zurita, Gaillard, Bottero and Hozbor.
Figures


Similar articles
-
Impact of maternal whole-cell or acellular pertussis primary immunization on neonatal immune response.Front Immunol. 2023 Jun 26;14:1192119. doi: 10.3389/fimmu.2023.1192119. eCollection 2023. Front Immunol. 2023. PMID: 37435078 Free PMC article.
-
Immunization with whole cell but not acellular pertussis vaccines primes CD4 TRM cells that sustain protective immunity against nasal colonization with Bordetella pertussis.Emerg Microbes Infect. 2019;8(1):169-185. doi: 10.1080/22221751.2018.1564630. Emerg Microbes Infect. 2019. PMID: 30866771 Free PMC article.
-
A Pertussis Outer Membrane Vesicle-Based Vaccine Induces Lung-Resident Memory CD4 T Cells and Protection Against Bordetella pertussis, Including Pertactin Deficient Strains.Front Cell Infect Microbiol. 2019 Apr 26;9:125. doi: 10.3389/fcimb.2019.00125. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31106160 Free PMC article.
-
Next-Generation Pertussis Vaccines Based on the Induction of Protective T Cells in the Respiratory Tract.Vaccines (Basel). 2020 Oct 21;8(4):621. doi: 10.3390/vaccines8040621. Vaccines (Basel). 2020. PMID: 33096737 Free PMC article. Review.
-
Comparison of adverse events following immunisation with acellular and whole-cell pertussis vaccines: A systematic review.Vaccine. 2018 Sep 25;36(40):6007-6016. doi: 10.1016/j.vaccine.2018.08.022. Epub 2018 Aug 22. Vaccine. 2018. PMID: 30143272
Cited by
-
Göttingen Minipigs as a Model to Evaluate Longevity, Functionality, and Memory of Immune Response Induced by Pertussis Vaccines.Front Immunol. 2021 Mar 17;12:613810. doi: 10.3389/fimmu.2021.613810. eCollection 2021. Front Immunol. 2021. PMID: 33815369 Free PMC article.
-
Intranasal challenge with B. pertussis leads to more severe disease manifestations in mice than aerosol challenge.PLoS One. 2023 Nov 2;18(11):e0286925. doi: 10.1371/journal.pone.0286925. eCollection 2023. PLoS One. 2023. PMID: 37917623 Free PMC article.
-
The Neonatal Immune System and Respiratory Pathogens.Microorganisms. 2023 Jun 16;11(6):1597. doi: 10.3390/microorganisms11061597. Microorganisms. 2023. PMID: 37375099 Free PMC article. Review.
-
Non-primate animal models for pertussis: back to the drawing board?Appl Microbiol Biotechnol. 2022 Feb;106(4):1383-1398. doi: 10.1007/s00253-022-11798-1. Epub 2022 Feb 1. Appl Microbiol Biotechnol. 2022. PMID: 35103810 Free PMC article. Review.
-
Impact of maternal whole-cell or acellular pertussis primary immunization on neonatal immune response.Front Immunol. 2023 Jun 26;14:1192119. doi: 10.3389/fimmu.2023.1192119. eCollection 2023. Front Immunol. 2023. PMID: 37435078 Free PMC article.
References
-
- Adkins B. (2005). Neonatal T cell function. J. Pediatr. Gastroenterol. Nutr. 40(Suppl. 1), S5–S7. - PubMed
-
- Agrawal A., Singh S., Kolhapure S., Kandeil W., Pai R., Singhal T. (2019). Neonatal pertussis, an under-recognized health burden and rationale for maternal immunization: a systematic review of South and South-East Asian Countries. Infect. Dis. Therapy 8 139–153. 10.1007/s40121-019-0245-2 - DOI - PMC - PubMed
-
- Allen A. C., Wilk M. M., Misiak A., Borkner L., Murphy D., Mills K. H. G. (2018). Sustained protective immunity against Bordetella pertussis nasal colonization by intranasal immunization with a vaccine-adjuvant combination that induces IL-17-secreting TRM cells. Mucosal Immunol. 11 1763–1776. 10.1038/s41385-018-0080-x - DOI - PubMed
-
- Asensio C. J., Gaillard M. E., Moreno G., Bottero D., Zurita E., Rumbo M., et al. (2011). Outer membrane vesicles obtained from Bordetella pertussis Tohama expressing the lipid A deacylase PagL as a novel acellular vaccine candidate. Vaccine 29 1649–1656. 10.1016/j.vaccine.2010.12.068 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

