Salmonella Enteritidis Effector AvrA Suppresses Autophagy by Reducing Beclin-1 Protein
- PMID: 32362899
- PMCID: PMC7181453
- DOI: 10.3389/fimmu.2020.00686
Salmonella Enteritidis Effector AvrA Suppresses Autophagy by Reducing Beclin-1 Protein
Abstract
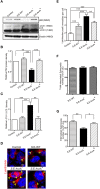
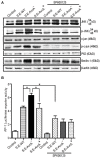
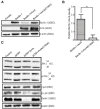
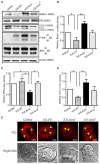
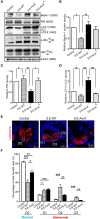
Autophagy is a cellular process to clear pathogens. Salmonella enterica serovar Enteritidis (S.E) has emerged as one of the most important food-borne pathogens. However, major studies still focus on Salmonella enterica serovar Typhimurium. Here, we reported that AvrA, a S. Enteritidis effector, inhibited autophagy to promote bacterial survival in the host. We found that AvrA regulates the conversion of LC3 I into LC3 II and the enrichment of lysosomes. Beclin-1, a key molecular regulator of autophagy, was decreased after AvrA expressed strain colonization. In S.E-AvrA--infected cells, we found the increases of protein levels of p-JNK and p-c-Jun and the transcription level of AP-1. AvrA-reduction of Beclin-1 protein expression is through the JNK pathway. The JNK inhibitor abolished the AvrA-reduced Beclin-1 protein expression. Moreover, we identified that the AvrA mutation C186A abolished its regulation of Beclin-1 expression. In addition AvrA protein was found interacted with Beclin-1. In organoids and infected mice, we explored the physiologically related effects and mechanism of AvrA in reducing Beclin-1 through the JNK pathway, thus attenuating autophagic responses. This finding not only indicates an important role of S. Enteritidis effector in reducing host protein as a strategy to suppress autophagy, but also suggests manipulating autophagy as a new strategy to treat infectious diseases.
Keywords: autophagy; effector; infection; organoids; paneth cells.
Copyright © 2020 Jiao, Zhang, Lin, Lu, Xia, Meng, Pan, Xu, Jiao and Sun.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

