Synthesis of organic liquid crystals containing selectively fluorinated cyclopropanes
- PMID: 32362945
- PMCID: PMC7176930
- DOI: 10.3762/bjoc.16.65
Synthesis of organic liquid crystals containing selectively fluorinated cyclopropanes
Abstract
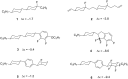
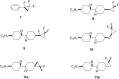
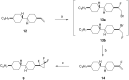
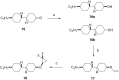
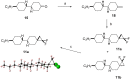
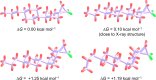
This paper describes the synthesis of a series of organic liquid crystals (LCs) containing selectively fluorinated cyclopropanes at their termini. The syntheses used difluorocarbene additions to olefin precursors, an approach which proved straightforward such that these liquid crystal candidates could be efficiently prepared. Their physical and thermodynamic properties were evaluated and depending on individual structures, they either displayed positive or negative dielectric anisotropy. The study gives some guidance into effective structure-property relationships for the design of LCs containing selectively fluorinated cyclopropane motifs.
Keywords: dielectric anisotropy; difluorocarbene; organic liquid crystals; selectively fluorinated cyclopropanes.
Copyright © 2020, Fang et al.; licensee Beilstein-Institut.
Figures
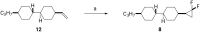
Similar articles
-
Fluorinated liquid crystals: evaluation of selectively fluorinated facially polarised cyclohexyl motifs for liquid crystal applications.Org Biomol Chem. 2016 Oct 25;14(42):9974-9980. doi: 10.1039/c6ob01986b. Org Biomol Chem. 2016. PMID: 27722421
-
Dielectric spectroscopy analysis in employing liquid crystal phthalonitrile derivative in nematic liquid crystals.Spectrochim Acta A Mol Biomol Spectrosc. 2007 Jun;67(2):531-5. doi: 10.1016/j.saa.2006.08.010. Epub 2006 Sep 12. Spectrochim Acta A Mol Biomol Spectrosc. 2007. PMID: 16971169
-
Fluorinated liquid crystals--properties and applications.Chem Soc Rev. 2007 Dec;36(12):2070-95. doi: 10.1039/b610738a. Epub 2007 Sep 14. Chem Soc Rev. 2007. PMID: 17982522 Review.
-
Design, synthesis and evaluation of new fluorinated liquid crystals bearing a CF2CF2 fragment with negative dielectric anisotropy.Org Biomol Chem. 2017 Feb 7;15(6):1495-1509. doi: 10.1039/c6ob02431a. Org Biomol Chem. 2017. PMID: 28116381
-
Recent advances in the total synthesis of cyclopropane-containing natural products.Chem Soc Rev. 2012 Jul 7;41(13):4631-42. doi: 10.1039/c2cs35067j. Epub 2012 May 16. Chem Soc Rev. 2012. PMID: 22592592 Review.
Cited by
-
The preparation and properties of 1,1-difluorocyclopropane derivatives.Beilstein J Org Chem. 2021 Jan 26;17:245-272. doi: 10.3762/bjoc.17.25. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 33564335 Free PMC article. Review.
-
Supramolecular packing of alkyl substituted Janus face all-cis 2,3,4,5,6-pentafluorocyclohexyl motifs.Chem Sci. 2021 Jun 4;12(28):9712-9719. doi: 10.1039/d1sc02130c. eCollection 2021 Jul 21. Chem Sci. 2021. PMID: 34349942 Free PMC article.
References
-
- Kirsch P. Modern Fluoroorganic Chemistry. 2nd ed. Weinheim, Germany: Wiley-VCH; 2013. - DOI
LinkOut - more resources
Full Text Sources
