Recent advances in Cu-catalyzed C(sp3)-Si and C(sp3)-B bond formation
- PMID: 32362947
- PMCID: PMC7176932
- DOI: 10.3762/bjoc.16.67
Recent advances in Cu-catalyzed C(sp3)-Si and C(sp3)-B bond formation
Abstract
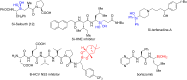
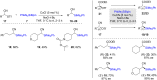
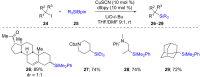
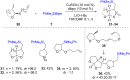
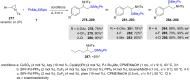
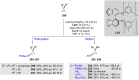
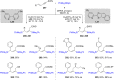
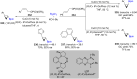
Numerous reactions generating C-Si and C-B bonds are in focus owing to the importance of incorporating silicon or boron into new or existing drugs, in addition to their use as building blocks in cross-coupling reactions en route to various targets of both natural and unnatural origins. In this review, recent protocols relying on copper-catalyzed sp3 carbon-silicon and carbon-boron bond-forming reactions are discussed.
Keywords: C–B bonds; C–Si bonds; copper catalysis; enantioselective reactions; sp3 carbon functionalization.
Copyright © 2020, Takale et al.; licensee Beilstein-Institut.
Figures



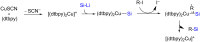
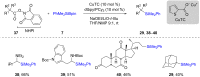
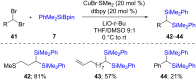




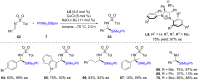
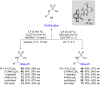

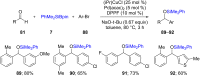



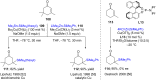
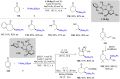


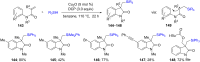




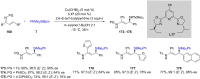
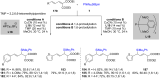



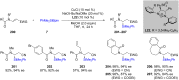









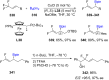


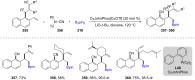
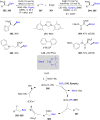

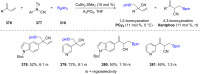


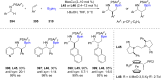





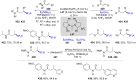

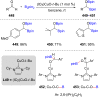
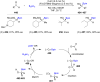





References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
