Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
- PMID: 32362948
- PMCID: PMC7176922
- DOI: 10.3762/bjoc.16.68
Combining enyne metathesis with long-established organic transformations: a powerful strategy for the sustainable synthesis of bioactive molecules
Abstract
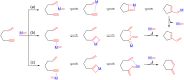
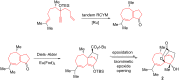
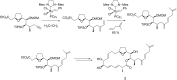
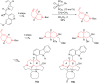
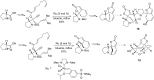
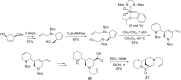
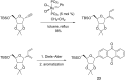
This account surveys the current progress on the application of intra- and intermolecular enyne metathesis as main key steps in the synthesis of challenging structural motifs and stereochemistries found in bioactive compounds. Special emphasis is placed on ruthenium catalysts as promoters of enyne metathesis to build the desired 1,3-dienic units. The advantageous association of this approach with name reactions like Grignard, Wittig, Diels-Alder, Suzuki-Miyaura, Heck cross-coupling, etc. is illustrated. Examples unveil the generality of such tandem reactions in providing not only the intricate structures of known, in vivo effective substances but also for designing chemically modified analogs as valid alternatives for further therapeutic agents.
Keywords: bioactive compounds; enyne metathesis; ring-closing metathesis; ruthenium catalysts; tandem reactions.
Copyright © 2020, Dragutan et al.; licensee Beilstein-Institut.
Figures
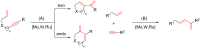














References
-
- Grubbs R H, Wenzel A G, O'Leary D J, et al., editors. Handbook of Metathesis. Weinheim, Germany: Wiley-VCH; 2015. - DOI
-
- Grela K, editor. Olefin Metathesis: Theory and Practice. Hoboken, New Jersey: John Wiley & Sons; 2014. - DOI
-
- Cheng-Sánchez I, Sarabia F. Synthesis. 2018;50:3749–3786. doi: 10.1055/s-0037-1610206. - DOI
Publication types
LinkOut - more resources
Full Text Sources
