Feeding Rhythms and the Circadian Regulation of Metabolism
- PMID: 32363197
- PMCID: PMC7182033
- DOI: 10.3389/fnut.2020.00039
Feeding Rhythms and the Circadian Regulation of Metabolism
Abstract
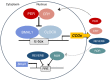
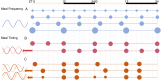
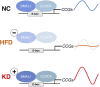
The molecular circadian clock regulates metabolic processes within the cell, and the alignment of these clocks between tissues is essential for the maintenance of metabolic homeostasis. The possibility of misalignment arises from the differential responsiveness of tissues to the environmental cues that synchronize the clock (zeitgebers). Although light is the dominant environmental cue for the master clock of the suprachiasmatic nucleus, many other tissues are sensitive to feeding and fasting. When rhythms of feeding behavior are altered, for example by shift work or the constant availability of highly palatable foods, strong feedback is sent to the peripheral molecular clocks. Varying degrees of phase shift can cause the systemic misalignment of metabolic processes. Moreover, when there is a misalignment between the endogenous rhythms in physiology and environmental inputs, such as feeding during the inactive phase, the body's ability to maintain homeostasis is impaired. The loss of phase coordination between the organism and environment, as well as internal misalignment between tissues, can produce cardiometabolic disease as a consequence. The aim of this review is to synthesize the work on the mechanisms and metabolic effects of circadian misalignment. The timing of food intake is highlighted as a powerful environmental cue with the potential to destroy or restore the synchrony of circadian rhythms in metabolism.
Keywords: circadian; fasting; high fat diet; ketogenic diet; metabolism; peripheral clock; time-restricted feeding.
Copyright © 2020 Pickel and Sung.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Medical

