Survival benefit of lung transplantation compared with medical management and pulmonary rehabilitation for patients with end-stage COPD
- PMID: 32363207
- PMCID: PMC7184114
- DOI: 10.1183/23120541.00177-2019
Survival benefit of lung transplantation compared with medical management and pulmonary rehabilitation for patients with end-stage COPD
Abstract
Background: COPD patients account for a large proportion of lung transplants; lung transplantation survival benefit for COPD patients is not well established.
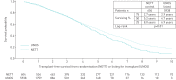
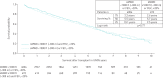
Methods: We identified 4521 COPD patients in the United Network for Organ Sharing (UNOS) dataset transplanted from May 2005 to August 2016, and 604 patients assigned to receive pulmonary rehabilitation and medical management in the National Emphysema Treatment Trial (NETT). After trimming the populations for NETT eligibility criteria and data completeness, 1337 UNOS and 596 NETT patients remained. Kaplan-Meier estimates of transplant-free survival from transplantation for UNOS, and NETT randomisation, were compared between propensity score-matched UNOS (n=401) and NETT (n=262) patients.
Results: In propensity-matched analyses, transplanted patients had better survival compared to medically managed patients in NETT (p=0.003). Stratifying on 6 min walk distance (6 MWD) and FEV1, UNOS patients with 6 MWD <1000 ft (∼300 m) or FEV1 <20% of predicted had better survival than NETT counterparts (median survival 5.0 years UNOS versus 3.4 years NETT; log-rank p<0.0001), while UNOS patients with 6 MWD ≥1000 ft (∼300 m) and FEV1 ≥20% had similar survival to NETT counterparts (median survival, 5.4 years UNOS versus 4.9 years NETT; log-rank p=0.73), interaction p=0.01.
Conclusions: Overall survival is better for matched lung transplant patients compared with medical management alone. Patients who derive maximum benefit are those with 6 MWD <1000 ft (∼300 m) or FEV1 <20% of predicted, compared with pulmonary rehabilitation and medical management.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: I. Timofte has nothing to disclose. Conflict of interest: M. Wijesinha has nothing to disclose. Conflict of interest: R. Vesselinov has nothing to disclose. Conflict of interest: J. Kim has nothing to disclose. Conflict of interest: R. Reed reports grants from the Dept of Defense and the Flight Attendant Medical Research Institute during the conduct of the study; and grants from the National Institutes of Health, the COPD Foundation, Janssen Research & Development LLC and the University of Maryland Institute for Clinical and Translational Research outside the submitted work. Conflict of interest: P.G. Sanchez has nothing to disclose. Conflict of interest: N. Ladikos has nothing to disclose. Conflict of interest: S. Pham has nothing to disclose. Conflict of interest: Z. Kon reports consulting and speaking fees from Medtronic, Inc., and consulting fees from Breethe, Inc., outside the submitted work. Conflict of interest: K. Rajagopal has nothing to disclose. Conflict of interest: S.M. Scharf has nothing to disclose. Conflict of interest: R. Wise reports grants, and personal fees for data monitoring committees and consulting from AstraZeneca, Medimmune, Pearl and Boehringer Ingelheim; personal fees for a clinical endpoint committee from Contrafect; personal fees for data safety monitoring committees from Pulmonx, Roche, Merck and AbbVie; personal fees for a steering committee from Spiration; personal fees for a workshop and consulting from Sunovion; research grants from Pearl Therapeutics and Sanofi-Aventis; personal fees for consultancy from Circassia, Pneuma, Verona, Mylan/Theravance and Propelleor Health; grants, and personal fees for a data monitoring committee, consultancy, a scientific advisory board and a clinical endpoint committee from GSK, outside the submitted work. Conflict of interest: A.L. Sternberg reports that the NETT was supported by contracts from the NHLBI during the conduct of the study. Conflict of interest: D. Kaczorowski has nothing to disclose. Conflict of interest: B. Griffith has nothing to disclose. Conflict of interest: M. Terrin has nothing to disclose. Conflict of interest: A. Iacono has nothing to disclose.
Figures


References
-
- Chambers DC, Yusen RD, Cherikh WS, et al. . The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2017; 36: 1047–1059, Epub 2017 Jul 19. doi:10.1016/j.healun.2017.07.016 - DOI - PubMed
Grants and funding
- N01 HR076103/HL/NHLBI NIH HHS/United States
- N01 HR076102/HL/NHLBI NIH HHS/United States
- N01 HR076114/HL/NHLBI NIH HHS/United States
- N01 HR076113/HL/NHLBI NIH HHS/United States
- N01 HR076104/HL/NHLBI NIH HHS/United States
- T32 AG000262/AG/NIA NIH HHS/United States
- N01 HR076101/HL/NHLBI NIH HHS/United States
- N01 HR076110/HL/NHLBI NIH HHS/United States
- N01 HR076112/HL/NHLBI NIH HHS/United States
- N01 HR076109/HL/NHLBI NIH HHS/United States
- N01 HR076105/HL/NHLBI NIH HHS/United States
- N01 HR076118/HL/NHLBI NIH HHS/United States
- N01 HR076115/HR/NHLBI NIH HHS/United States
- N01 HR076107/HL/NHLBI NIH HHS/United States
- N01 HR076106/HL/NHLBI NIH HHS/United States
- N01 HR076119/HL/NHLBI NIH HHS/United States
- N01 HR076108/HL/NHLBI NIH HHS/United States
- N01 HR076116/HL/NHLBI NIH HHS/United States
- N01 HR076111/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
