In Situ Absorption and Fluorescence Microspectroscopy Investigation of the Molecular Incorporation Process into Single Nanoporous Protein Crystals
- PMID: 32363313
- PMCID: PMC7191835
- DOI: 10.1021/acsomega.0c01038
In Situ Absorption and Fluorescence Microspectroscopy Investigation of the Molecular Incorporation Process into Single Nanoporous Protein Crystals
Abstract
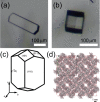
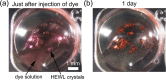
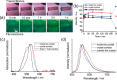
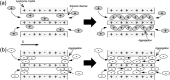
Protein crystals exhibit distinct three-dimensional structures, which contain well-ordered nanoporous solvent channels, providing a chemically heterogeneous environment. In this paper, the incorporation of various molecules into the solvent channels of native hen egg-white lysozyme crystals was demonstrated using fluorescent dyes, including acridine yellow G, rhodamine 6G, and eosin Y. The process was evaluated on the basis of absorption and fluorescence microspectroscopy at a single-crystal level. The molecular loading process was clearly visualized as a function of time, and it was determined that the protein crystals could act as nanoporous materials. It was found that the incorporation process is strongly dependent on the molecular charge, leading to heterogeneous molecular aggregation, which suggests host-guest interaction of protein crystals from the viewpoint of nanoporous materials.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Synthesis and confinement of carbon dots in lysozyme single crystals produces ordered hybrid materials with tuneable luminescence.Chemistry. 2015 Jun 15;21(25):9008-13. doi: 10.1002/chem.201501429. Epub 2015 May 8. Chemistry. 2015. PMID: 25959990
-
Adsorption of xanthene dyes by lysozyme crystals.Langmuir. 2005 Feb 15;21(4):1475-80. doi: 10.1021/la0478090. Langmuir. 2005. PMID: 15697297
-
Self-association of rhodamine dyes in different host materials.Spectrochim Acta A Mol Biomol Spectrosc. 2001 Aug;57(9):1865-71. doi: 10.1016/s1386-1425(01)00417-6. Spectrochim Acta A Mol Biomol Spectrosc. 2001. PMID: 11506038
-
Molecular simulations for energy, environmental and pharmaceutical applications of nanoporous materials: from zeolites, metal-organic frameworks to protein crystals.Chem Soc Rev. 2011 Jul;40(7):3599-612. doi: 10.1039/c0cs00128g. Epub 2011 Apr 21. Chem Soc Rev. 2011. PMID: 21512695 Review.
-
Atomic force microscopy in the study of macromolecular crystal growth.Annu Rev Biophys Biomol Struct. 2000;29:361-410. doi: 10.1146/annurev.biophys.29.1.361. Annu Rev Biophys Biomol Struct. 2000. PMID: 10940253 Review.
Cited by
-
Production of Cross-Linked Lipase Crystals at a Preparative Scale.Cryst Growth Des. 2021 Mar 3;21(3):1698-1707. doi: 10.1021/acs.cgd.0c01608. Epub 2021 Feb 17. Cryst Growth Des. 2021. PMID: 34602865 Free PMC article.
-
Magnetite Mineralization inside Cross-Linked Protein Crystals.Cryst Growth Des. 2023 Apr 28;23(6):4032-4040. doi: 10.1021/acs.cgd.2c01436. eCollection 2023 Jun 7. Cryst Growth Des. 2023. PMID: 37304398 Free PMC article.
References
-
- Vilenchik L. Z.; Griffith J. P.; Clair N.; Navia M. A.; Margolin A. L. Protein Crystals as Novel Microporous Materials. J. Am. Chem. Soc. 1998, 120, 4290–4294. 10.1021/ja973449+. - DOI
-
- Brühwiler D.; Calzaferri G.; Torres T.; Ramm J. H.; Gartmann N.; Dieu L.-Q.; López-Duarte I.; Martínez-Díaz M. V. Nanochannels for Supramolecular Organization of Luminescent Guests. J. Mater. Chem. 2009, 19, 8040–8067. 10.1039/b907308f. - DOI
LinkOut - more resources
Full Text Sources

