Molecular Mechanism for Protection Against Liver Failure in Human Yellow Fever Infection
- PMID: 32363317
- PMCID: PMC7193135
- DOI: 10.1002/hep4.1504
Molecular Mechanism for Protection Against Liver Failure in Human Yellow Fever Infection
Abstract
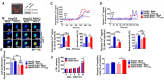
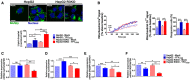
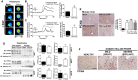
Yellow fever (YF) is a viral hemorrhagic fever that typically involves the liver. Brazil recently experienced its largest recorded YF outbreak, and the disease was fatal in more than a third of affected individuals, mostly because of acute liver failure. Affected individuals are generally treated only supportively, but during the recent Brazilian outbreak, selected patients were treated with liver transplant. We took advantage of this clinical experience to better characterize the clinical and pathological features of YF-induced liver failure and to examine the mechanism of hepatocellular injury in YF, to identify targets that would be amenable to therapeutic intervention in preventing progression to liver failure and death. Patients with YF liver failure rapidly developed massive transaminase elevations, with jaundice, coagulopathy, thrombocytopenia, and usually hepatic encephalopathy, along with pathological findings that included microvesicular steatosis and lytic necrosis. Hepatocytes began to express the type 3 isoform of the inositol trisphosphate receptor (ITPR3), an intracellular calcium (Ca2+) channel that is not normally expressed in hepatocytes. Experiments in an animal model, isolated hepatocytes, and liver-derived cell lines showed that this new expression of ITPR3 was associated with increased nuclear Ca2+ signaling and hepatocyte proliferation, and reduced steatosis and cell death induced by the YF virus. Conclusion: Yellow fever often induces liver failure characterized by massive hepatocellular damage plus steatosis. New expression of ITPR3 also occurs in YF-infected hepatocytes, which may represent an endogenous protective mechanism that could suggest approaches to treat affected individuals before they progress to liver failure, thereby decreasing the mortality of this disease in a way that does not rely on the costly and limited resource of liver transplantation.
© 2020 The Authors. Hepatology Communications published by Wiley Periodicals, Inc., on behalf of the American Association for the Study of Liver Diseases.
Figures


References
-
- Barrett AD, Monath TP. Epidemiology and ecology of yellow fever virus. Adv Virus Res 2003;61:291‐315. - PubMed
-
- Monath TP. Yellow fever: an update. Lancet Infect Dis 2001;1:11‐20. - PubMed
-
- Kallas EG, D'Elia Zanella L, Moreira CHV, Buccheri R, Diniz GBF, Castineiras ACP, et al. Predictors of mortality in patients with yellow fever: an observational cohort study. Lancet Infect Dis 2019;19:750‐758. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

