Gene Transfer in Rodent Nervous Tissue Following Hindlimb Intramuscular Delivery of Recombinant Adeno-Associated Virus Serotypes AAV2/6, AAV2/8, and AAV2/9
- PMID: 32363345
- PMCID: PMC7176396
- DOI: 10.1177/1179069519889022
Gene Transfer in Rodent Nervous Tissue Following Hindlimb Intramuscular Delivery of Recombinant Adeno-Associated Virus Serotypes AAV2/6, AAV2/8, and AAV2/9
Abstract
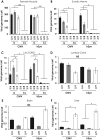
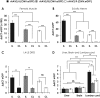
Recombinant adeno-associated virus (rAAV) vectors have emerged as the safe vehicles of choice for long-term gene transfer in mammalian nervous system. Recombinant adeno-associated virus-mediated localized gene transfer in adult nervous system following direct inoculation, that is, intracerebral or intrathecal, is well documented. However, recombinant adeno-associated virus delivery in defined neuronal populations in adult animals using less-invasive methods as well as avoiding ectopic gene expression following systemic inoculation remain challenging. Harnessing the capability of some recombinant adeno-associated virus serotypes for retrograde transduction may potentially address such limitations (Note: The term retrograde transduction in this manuscript refers to the uptake of injected recombinant adeno-associated virus particles at nerve terminals, retrograde transport, and subsequent transduction of nerve cell soma). In some studies, recombinant adeno-associated virus serotypes 2/6, 2/8, and 2/9 have been shown to exhibit transduction of connected neuroanatomical tracts in adult animals following lower limb intramuscular recombinant adeno-associated virus delivery in a pattern suggestive of retrograde transduction. However, an extensive side-by-side comparison of these serotypes following intramuscular delivery regarding tissue viral load, and the effect of promoter on transgene expression, has not been performed. Hence, we delivered recombinant adeno-associated virus serotypes 2/6, 2/8, or 2/9 encoding enhanced green fluorescent protein (eGFP), under the control of either cytomegalovirus (CMV) or human synapsin (hSyn) promoter, via a single unilateral hindlimb intramuscular injection in the bicep femoris of adult C57BL/6J mice. Four weeks post injection, we quantified viral load and transgene (enhanced green fluorescent protein) expression in muscle and related nervous tissues. Our data show that the select recombinant adeno-associated virus serotypes transduce sciatic nerve and groups of neurons in the dorsal root ganglia on the injected side, indicating that the intramuscular recombinant adeno-associated virus delivery is useful for achieving gene transfer in local neuroanatomical tracts. We also observed sparse recombinant adeno-associated virus viral delivery or eGFP transduction in lumbar spinal cord and a noticeable lack thereof in brain. Therefore, further improvements in recombinant adeno-associated virus design are warranted to achieve efficient widespread retrograde transduction following intramuscular and possibly other peripheral routes of delivery.
Keywords: Adeno-associated virus; gene delivery; nervous system; recombinant adeno-associated virus delivery.
© The Author(s) 2019.
Conflict of interest statement
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures





References
-
- Bedbrook CN, Deverman BE, Gradinaru V. Viral strategies for targeting the central and peripheral nervous systems. Annu Rev Neurosci. 2018;41:323-348. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

